Oral delivery of human biopharmaceuticals, autoantigens and vaccine antigens bioencapsulated in plant cells
- PMID: 23099275
- PMCID: PMC3582797
- DOI: 10.1016/j.addr.2012.10.005
Oral delivery of human biopharmaceuticals, autoantigens and vaccine antigens bioencapsulated in plant cells
Abstract
Among 12billion injections administered annually, unsafe delivery leads to >20million infections and >100million reactions. In an emerging new concept, freeze-dried plant cells (lettuce) expressing vaccine antigens/biopharmaceuticals are protected in the stomach from acids/enzymes but are released to the immune or blood circulatory system when plant cell walls are digested by microbes that colonize the gut. Vaccine antigens bioencapsulated in plant cells upon oral delivery after priming, conferred both mucosal and systemic immunity and protection against bacterial, viral or protozoan pathogens or toxin challenge. Oral delivery of autoantigens was effective against complications of type 1 diabetes and hemophilia, by developing tolerance. Oral delivery of proinsulin or exendin-4 expressed in plant cells regulated blood glucose levels similar to injections. Therefore, this new platform offers a low cost alternative to deliver different therapeutic proteins to combat infectious or inherited diseases by eliminating inactivated pathogens, expensive purification, cold storage/transportation and sterile injections.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures


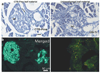
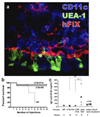


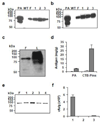
References
-
- Engaging Communities to Improve Global health: Reducing Disease Burden through Collaborative Approaches; Johannesburg and Durban; 2010. http://conferences.thehillgroup.com/obssr/engagingcommunities/about.html.
-
- Nitesh SC, Sanjeev C, Vandana H, Alka A, Vijender S. Recent advances in insulin delivery systems: an update. World Applied Sciences Journal. 2010;11:1552–1556.
-
- Kermode M. Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Health Promot. Int. 2004;19:95–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

