Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions
- PMID: 23099485
- PMCID: PMC3571618
- DOI: 10.1038/nprot.2012.130
Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions
Abstract
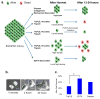
This protocol describes an EDTA-based passaging procedure to be used with chemically defined E8 medium that serves as a tool for basic and translational research into human pluripotent stem cells (PSCs). In this protocol, passaging one six-well or 10-cm plate of cells takes about 6-7 min. This enzyme-free protocol achieves maximum cell survival without enzyme neutralization, centrifugation or drug treatment. It also allows for higher throughput, requires minimal material and limits contamination. Here we describe how to produce a consistent E8 medium for routine maintenance and reprogramming and how to incorporate the EDTA-based passaging procedure into human induced PSC (iPSC) derivation, colony expansion, cryopreservation and teratoma formation. This protocol has been successful in routine cell expansion, and efficient for expanding large-volume cultures or a large number of cells with preferential dissociation of PSCs. Effective for all culture stages, this procedure provides a consistent and universal approach to passaging human PSCs in E8 medium.
Conflict of interest statement
J.A.T. is a founder, stockowner, consultant, and board member of Cellular Dynamics International. He also serves as scientific advisor to and has financial interests in Tactics II Stem Cell Ventures.
Figures

References
-
- Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. - PubMed
-
- Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. - PubMed
-
- Yu JY, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. - PubMed
-
- Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

