Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: results of Children's Oncology Group trial A9961
- PMID: 23099653
- PMCID: PMC3534419
- DOI: 10.1093/neuonc/nos267
Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: results of Children's Oncology Group trial A9961
Abstract
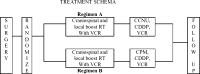
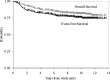
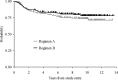
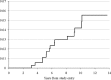
The purpose of the trial was to determine the survival and incidence of secondary tumors in children with medulloblastoma receiving radiotherapy plus chemotherapy. Three hundred seventy-nine eligible patients with nondisseminated medulloblastoma between the ages of 3 and 21 years were treated with 2340 cGy of craniospinal and 5580 cGy of posterior fossa irradiation. Patients were randomized between postradiation cisplatin and vincristine plus either CCNU or cyclophosphamide. Survival, pattern of relapse, and occurrence of secondary tumors were assessed. Five- and 10-year event-free survivals were 81 ± 2% and 75.8 ± 2.3%; overall survivals were 87 ± 1.8% and 81.3 ± 2.1%. Event-free survival was not impacted by chemotherapeutic regimen, sex, race, age at diagnosis, or gender. Seven patients had disease relapse beyond 5 years after diagnosis; relapse was local in 4 patients, local plus supratentorial in 2, and supratentorial alone in 1. Fifteen patients experienced secondary tumors as a first event at a median time of 5.8 years after diagnosis (11 >5 y postdiagnosis). All non-CNS solid secondary tumors (4) occurred in regions that had received radiation. Of the 6 high-grade gliomas, 5 occurred >5 years postdiagnosis. The estimated cumulative 10-year incidence rate of secondary malignancies was 4.2% (1.9%-6.5%). Few patients with medulloblastoma will relapse ≥ 5 years postdiagnosis; relapse will occur predominantly at the primary tumor site. Patients are at risk for development of secondary tumors, many of which are malignant gliomas. This may become an increasing issue as more children survive.
Figures
References
-
- Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24(25):4202–4203. doi:10.1200/JCO.2006.06.4980. - DOI - PubMed
-
- Oyharcabal-Bourden V, Kalifa C, Gentet JC, et al. Standard-risk medulloblastoma treated by adjuvant chemotherapy followed by reduced-dose craniospinal radiation therapy: a French Society of Pediatric Oncology study. J Clin Oncol. 2005;23(19):4726–4734. doi:10.1200/JCO.2005.00.760. - DOI - PubMed
-
- Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. http://oncology.thelancet.com. Published online September 7, 2006. doi:10.1016/S1470-2045(06)70867-1. - DOI - PubMed
-
- Thomas PRM, Deutsch M, Kepner JL, et al. Low-stage medulloblastoma: final analysis of trial comparing standard-dose with reduced-dose neuroaxis irradiation. J Clin Oncol. 2000;18(16):3004–3011. - PubMed
-
- Taylor RE, Bailey CC, Robinson K, et al. Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: the International Society of Paediatric Oncology/United Kingdom Children's Cancer Study Group PNET-3 study. J Clin Oncol. 2003;21(8):1581–1591. doi:10.1200/JCO.2003.05.116. - DOI - PubMed