Ectopic expression of GIP in pancreatic β-cells maintains enhanced insulin secretion in mice with complete absence of proglucagon-derived peptides
- PMID: 23099862
- PMCID: PMC3554360
- DOI: 10.2337/db12-0294
Ectopic expression of GIP in pancreatic β-cells maintains enhanced insulin secretion in mice with complete absence of proglucagon-derived peptides
Abstract
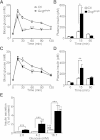
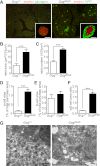
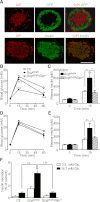
Glucagon and glucagon-like peptide-1 (GLP-1) are produced in pancreatic α-cells and enteroendocrine L-cells, respectively, in a tissue-specific manner from the same precursor, proglucagon, that is encoded by glucagon gene (Gcg), and play critical roles in glucose homeostasis. Here, we studied glucose homeostasis and β-cell function of Gcg-deficient mice that are homozygous for a Gcg-GFP knock-in allele (Gcg(gfp/gfp)). The Gcg(gfp/gfp) mice displayed improved glucose tolerance and enhanced insulin secretion, as assessed by both oral glucose tolerance test (OGTT) and intraperitoneal glucose tolerance test (IPGTT). Responses of glucose-dependent insulinotropic polypeptide (GIP) to both oral and intraperitoneal glucose loads were unexpectedly enhanced in Gcg(gfp/gfp) mice, and immunohistochemistry localized GIP to pancreatic β-cells of Gcg(gfp/gfp) mice. Furthermore, secretion of GIP in response to glucose was detected in isolated islets of Gcg(gfp/gfp) mice. Blockade of GIP action in vitro and in vivo by cAMP antagonism and genetic deletion of the GIP receptor, respectively, almost completely abrogated enhanced insulin secretion in Gcg(gfp/gfp) mice. These results indicate that ectopic GIP expression in β-cells maintains insulin secretion in the absence of proglucagon-derived peptides (PGDPs), revealing a novel compensatory mechanism for sustaining incretin hormone action in islets.
Figures


References
-
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–2157 - PubMed
-
- Hayashi Y. Metabolic impact of glucagon deficiency. Diabetes Obes Metab 2011;13(Suppl 1):151–157 - PubMed
-
- Unger RH. Glucagon physiology and pathophysiology in the light of new advances. Diabetologia 1985;28:574–578 - PubMed
-
- Jiang G, Zhang BB. Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab 2003;284:E671–E678 - PubMed
-
- Cherrington AD, Chiasson JL, Liljenquist JE, Lacy WW, Park CR. Control of hepatic glucose output by glucagon and insulin in the intact dog. Biochem Soc Symp 1978;43:31–45 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

