A critical role for Telethonin in regulating t-tubule structure and function in the mammalian heart
- PMID: 23100327
- PMCID: PMC3526164
- DOI: 10.1093/hmg/dds434
A critical role for Telethonin in regulating t-tubule structure and function in the mammalian heart
Abstract
The transverse (t)-tubule system plays an essential role in healthy and diseased heart muscle, particularly in Ca(2+)-induced Ca(2+) release (CICR), and its structural disruption is an early event in heart failure. Both mechanical overload and unloading alter t-tubule structure, but the mechanisms mediating the normally tight regulation of the t-tubules in response to load variation are poorly understood. Telethonin (Tcap) is a stretch-sensitive Z-disc protein that binds to proteins in the t-tubule membrane. To assess its role in regulating t-tubule structure and function, we used Tcap knockout (KO) mice and investigated cardiomyocyte t-tubule and cell structure and CICR over time and following mechanical overload. In cardiomyocytes from 3-month-old KO (3mKO), there were isolated t-tubule defects and Ca(2+) transient dysynchrony without whole heart and cellular dysfunction. Ca(2+) spark frequency more than doubled in 3mKO. At 8 months of age (8mKO), cardiomyocytes showed progressive loss of t-tubules and remodelling of the cell surface, with prolonged and dysynchronous Ca(2+) transients. Ca(2+) spark frequency was elevated and the L-type Ca(2+) channel was depressed at 8 months only. After mechanical overload obtained by aortic banding constriction, the Ca(2+) transient was prolonged in both wild type and KO. Mechanical overload increased the Ca(2+) spark frequency in KO alone, where there was also significantly more t-tubule loss, with a greater deterioration in t-tubule regularity. In conjunction, Tcap KO showed severe loss of cell surface ultrastructure. These data suggest that Tcap is a critical, load-sensitive regulator of t-tubule structure and function.
Figures


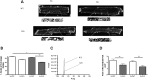
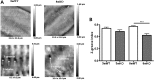



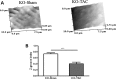
Similar articles
-
Cardiomyocyte Ca2+ handling and structure is regulated by degree and duration of mechanical load variation.J Cell Mol Med. 2012 Dec;16(12):2910-8. doi: 10.1111/j.1582-4934.2012.01611.x. J Cell Mol Med. 2012. PMID: 22862818 Free PMC article.
-
Microtubule-mediated defects in junctophilin-2 trafficking contribute to myocyte transverse-tubule remodeling and Ca2+ handling dysfunction in heart failure.Circulation. 2014 Apr 29;129(17):1742-50. doi: 10.1161/CIRCULATIONAHA.113.008452. Epub 2014 Feb 11. Circulation. 2014. PMID: 24519927 Free PMC article.
-
Mechanical unloading reverses transverse tubule remodelling and normalizes local Ca(2+)-induced Ca(2+)release in a rodent model of heart failure.Eur J Heart Fail. 2012 Jun;14(6):571-80. doi: 10.1093/eurjhf/hfs038. Epub 2012 Apr 1. Eur J Heart Fail. 2012. PMID: 22467752 Free PMC article.
-
BIN1 regulates dynamic t-tubule membrane.Biochim Biophys Acta. 2016 Jul;1863(7 Pt B):1839-47. doi: 10.1016/j.bbamcr.2015.11.004. Epub 2015 Nov 11. Biochim Biophys Acta. 2016. PMID: 26578114 Free PMC article. Review.
-
A functional role for transverse (t-) tubules in the atria.J Mol Cell Cardiol. 2013 May;58:84-91. doi: 10.1016/j.yjmcc.2012.11.001. Epub 2012 Nov 9. J Mol Cell Cardiol. 2013. PMID: 23147188 Review.
Cited by
-
Targeting cardiomyocyte Ca2+ homeostasis in heart failure.Curr Pharm Des. 2015;21(4):431-48. doi: 10.2174/138161282104141204124129. Curr Pharm Des. 2015. PMID: 25483944 Free PMC article. Review.
-
Constitutive deletion of the obscurin-Ig58/59 domains induces atrial remodeling and Ca2+-based arrhythmogenesis.JCI Insight. 2025 Jan 7;10(4):e184202. doi: 10.1172/jci.insight.184202. JCI Insight. 2025. PMID: 39804820 Free PMC article.
-
Elevated ventricular wall stress disrupts cardiomyocyte t-tubule structure and calcium homeostasis.Cardiovasc Res. 2016 Oct;112(1):443-51. doi: 10.1093/cvr/cvw111. Epub 2016 May 25. Cardiovasc Res. 2016. PMID: 27226008 Free PMC article.
-
Circadian regulation of myocardial sarcomeric Titin-cap (Tcap, telethonin): identification of cardiac clock-controlled genes using open access bioinformatics data.PLoS One. 2014 Aug 14;9(8):e104907. doi: 10.1371/journal.pone.0104907. eCollection 2014. PLoS One. 2014. PMID: 25121604 Free PMC article.
-
Microdomain-specific localization of functional ion channels in cardiomyocytes: an emerging concept of local regulation and remodelling.Biophys Rev. 2015 Mar;7(1):43-62. doi: 10.1007/s12551-014-0159-x. Epub 2015 Jan 15. Biophys Rev. 2015. PMID: 28509981 Free PMC article.
References
-
- Ibrahim M., Al Masri A., Navaratnarajah M., Siedlecka U., Soppa G.K., Moshkov A., Al-Saud S.A., Gorelik J., Yacoub M.H., Terracciano C.M. Prolonged mechanical unloading affects cardiomyocyte excitation-contraction coupling, transverse-tubule structure, and the cell surface. FASEB J. 2010;24:3321–3329. - PMC - PubMed
-
- Ibrahim M., Navaratnarajah M., Siedlecka U., Rao C., Dias P., Moshkov A.V., Gorelik J., Yacoub M.H., Terracciano C.M. Mechanical unloading reverses transverse tubule remodelling and normalizes local Ca(2+)-induced Ca(2+) release in a rodent model of heart failure. Eur. J. Heart Fail. 2012;14:571–580. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

