A novel, sensitive assay for high-throughput molecular detection of plasmodia for active screening of malaria for elimination
- PMID: 23100347
- PMCID: PMC3536241
- DOI: 10.1128/JCM.02010-12
A novel, sensitive assay for high-throughput molecular detection of plasmodia for active screening of malaria for elimination
Abstract
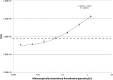
Although malaria remains one of the leading infectious diseases in the world, the decline in malaria transmission in some area makes it possible to consider elimination of the disease. As countries approach elimination, malaria diagnosis needs to change from diagnosing ill patients to actively detecting infections in all carriers, including asymptomatic and low-parasite-load patients. However, few of the current diagnostic methods have both the throughput and the sensitivity required. We adopted a sandwich RNA hybridization assay to detect genus Plasmodium 18S rRNA directly from whole-blood samples from Plasmodium falciparum and Plasmodium vivax patients without RNA isolation. We tested the assay with 202 febrile patients from areas where malaria is endemic, using 20 μl of each blood sample in a 96-well plate format with a 2-day enzyme-linked immunosorbent assay (ELISA)-like work flow. The results were compared with diagnoses obtained using microscopy, a rapid diagnostic test (RDT), and genus-specific real-time PCR. Our assay identified all 66 positive samples diagnosed by microscopy, including 49 poorly stored samples that underwent multiple freeze-thaw cycles due to resource limitation. The assay uncovered three false-negative samples by microscopy and four false-negative samples by RDT and agreed completely with real-time PCR diagnosis. There was no negative sample by our assay that would show a positive result when tested with other methods. The detection limit of our assay for P. falciparum was 0.04 parasite/μl. The assay's simple work flow, high throughput, and sensitivity make it suitable for active malaria screening.
Figures

References
-
- Harris I, Sharrock WW, Bain LM, Gray KA, Bobogare A, Boaz L, Lilley K, Krause D, Vallely A, Johnson ML, Gatton ML, Shanks GD, Cheng Q. 2010. A large proportion of asymptomatic Plasmodium infections with low and sub-microscopic parasite densities in the low transmission setting of Temotu Province, Solomon Islands: challenges for malaria diagnostics in an elimination setting. Malar. J. 9:254. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

