Neurofascin as a target for autoantibodies in peripheral neuropathies
- PMID: 23100406
- PMCID: PMC3542349
- DOI: 10.1212/WNL.0b013e31827689ad
Neurofascin as a target for autoantibodies in peripheral neuropathies
Abstract
Objectives: We asked whether autoantibodies against neurofascin (NF)186 or NF155, both localized at the nodes of Ranvier, are present in serum of patients with inflammatory neuropathy, and whether NF-specific monoclonal antibodies are pathogenic in vivo.
Methods: We cloned human NF155 and NF186, and developed an ELISA and cell-based assay to screen for antibodies to human NF in a total of 434 donors including 294 patients with Guillain-Barré syndrome variants acute inflammatory demyelinating polyneuropathy (AIDP), acute motor axonal neuropathy, and chronic inflammatory demyelinating polyneuropathy (CIDP). We characterized reactive samples by isotyping, tissue section staining, and epitope mapping. We also injected NF-specific monoclonal antibodies IV into rats with experimental autoimmune neuritis.
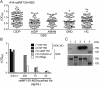
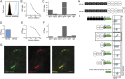
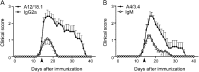
Results: We detected autoantibodies to NF by ELISA in 4% of patients with AIDP and CIDP, but not in controls. Most positive samples contained immunoglobulin G (IgG)1, IgG3, or IgG4 antibodies directed to only one isoform of NF. Two patients with CIDP showed particularly high (1:10,000 dilution) NF155-specific reactivity in both assays and stained paranodes. Two other patients with CIDP who benefited from plasma exchange exhibited antibodies to NF155 by ELISA, and upon affinity purification, antibodies to both isoforms were observed by both assays. Anti-NF monoclonal antibodies enhanced and prolonged induced neuritis in rats.
Conclusions: Autoantibodies to NF are detected in a very small proportion of patients with AIDP and patients with CIDP, but may nevertheless be pathogenic in these cases.
Figures

Comment in
-
Neurofascin antibodies in inflammatory neuropathy: how many needles make a haystack?Neurology. 2012 Dec 4;79(23):2224-5. doi: 10.1212/WNL.0b013e3182768b55. Epub 2012 Oct 24. Neurology. 2012. PMID: 23100403 No abstract available.
References
-
- Hughes RAC, Cornblath DR. Guillain-Barré syndrome. Lancet 2005;366:1653–1666 - PubMed
-
- Willison HJ, Yuki N. Peripheral neuropathies and anti-glycolipid antibodies. Brain 2002;125:2591–2625 - PubMed
-
- Kaida K, Kusunoki S. Antibodies to gangliosides and ganglioside complexes in Guillain-Barré syndrome and Fisher syndrome: mini-review. J Neuroimmunol 2010;223:5–12 - PubMed
-
- Illa I, Ortiz N, Gallard E, et al. Acute axonal Guillain-Barré syndrome with IgG antibodies against motor axons following parenteral gangliosides. Ann Neurol 1995;38:218–224 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
