Autism-associated mutations in ProSAP2/Shank3 impair synaptic transmission and neurexin-neuroligin-mediated transsynaptic signaling
- PMID: 23100419
- PMCID: PMC3752148
- DOI: 10.1523/JNEUROSCI.2215-12.2012
Autism-associated mutations in ProSAP2/Shank3 impair synaptic transmission and neurexin-neuroligin-mediated transsynaptic signaling
Abstract
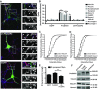
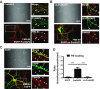
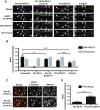
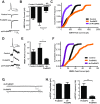
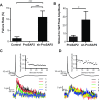
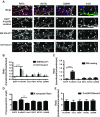
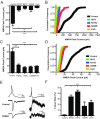
Mutations in several postsynaptic proteins have recently been implicated in the molecular pathogenesis of autism and autism spectrum disorders (ASDs), including Neuroligins, Neurexins, and members of the ProSAP/Shank family, thereby suggesting that these genetic forms of autism may share common synaptic mechanisms. Initial studies of ASD-associated mutations in ProSAP2/Shank3 support a role for this protein in glutamate receptor function and spine morphology, but these synaptic phenotypes are not universally penetrant, indicating that other core facets of ProSAP2/Shank3 function must underlie synaptic deficits in patients with ASDs. In the present study, we have examined whether the ability of ProSAP2/Shank3 to interact with the cytoplasmic tail of Neuroligins functions to coordinate pre/postsynaptic signaling through the Neurexin-Neuroligin signaling complex in hippocampal neurons of Rattus norvegicus. Indeed, we find that synaptic levels of ProSAP2/Shank3 regulate AMPA and NMDA receptor-mediated synaptic transmission and induce widespread changes in the levels of presynaptic and postsynaptic proteins via Neurexin-Neuroligin transsynaptic signaling. ASD-associated mutations in ProSAP2/Shank3 disrupt not only postsynaptic AMPA and NMDA receptor signaling but also interfere with the ability of ProSAP2/Shank3 to signal across the synapse to alter presynaptic structure and function. These data indicate that ASD-associated mutations in a subset of synaptic proteins may target core cellular pathways that coordinate the functional matching and maturation of excitatory synapses in the CNS.
Figures
References
-
- Bailey A, Phillips W, Rutter M. Autism: towards an integration of clinical, genetic, neuropsychological, and neurobiological perspectives. J Child Psychol Psychiatry. 1996;37:89–126. - PubMed
-
- Bangash MA, Park JM, Melnikova T, Wang D, Jeon SK, Lee D, Syeda S, Kim J, Kouser M, Schwartz J, Cui Y, Zhao X, Speed HE, Kee SE, Tu JC, Hu JH, Petralia RS, Linden DJ, Powell CM, Savonenko A, Xiao B, Worley PF. Enhanced polyubiquitination of Shank3 and NMDA receptor in a mouse model of autism. Cell. 2011;145:758–772. - PMC - PubMed
-
- Berkel S, Marshall CR, Weiss B, Howe J, Roeth R, Moog U, Endris V, Roberts W, Szatmari P, Pinto D, Bonin M, Riess A, Engels H, Sprengel R, Scherer SW, Rappold GA. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat Genet. 2010;42:489–491. - PubMed
-
- Boeckers TM, Bockmann J, Kreutz MR, Gundelfinger ED. ProSAP/Shank proteins—a family of higher order organizing molecules of the postsynaptic density with an emerging role in human neurological disease. J Neurochem. 2002;81:903–910. - PubMed
-
- Bourgeron T. A synaptic trek to autism. Curr Opin Neurobiol. 2009;19:231–234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
