Ventral tegmental area glutamate neurons: electrophysiological properties and projections
- PMID: 23100428
- PMCID: PMC3685320
- DOI: 10.1523/JNEUROSCI.3128-12.2012
Ventral tegmental area glutamate neurons: electrophysiological properties and projections
Abstract
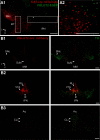
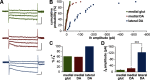
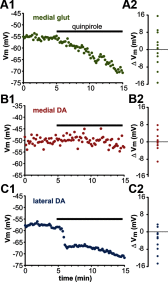
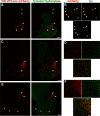
The ventral tegmental area (VTA) has a central role in the neural processes that underlie motivation and behavioral reinforcement. Although thought to contain only dopamine and GABA neurons, the VTA also includes a recently discovered population of glutamate neurons identified through the expression of the vesicular glutamate transporter VGLUT2. A subset of VGLUT2(+) VTA neurons corelease dopamine with glutamate at terminals in the NAc, but others do not express dopaminergic markers and remain poorly characterized. Using transgenic mice that express fluorescent proteins in distinct cell populations, we now find that both dopamine and glutamate neurons in the medial VTA exhibit a smaller hyperpolarization-activated current (I(h)) than more lateral dopamine neurons and less consistent inhibition by dopamine D(2) receptor agonists. In addition, VGLUT2(+) VTA neurons project to the nucleus accumbens (NAc), lateral habenula, ventral pallidum (VP), and amygdala. Optical stimulation of VGLUT2(+) projections expressing channelrhodopsin-2 further reveals functional excitatory synapses in the VP as well as the NAc. Thus, glutamate neurons form a physiologically and anatomically distinct subpopulation of VTA projection neurons.
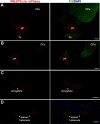
Figures

References
-
- Bäckman CM, Malik N, Zhang Y, Shan L, Grinberg A, Hoffer BJ, Westphal H, Tomac AC. Characterization of a mouse strain expressing Cre recombinase from the 3′ untranslated region of the dopamine transporter locus. Genesis. 2006;44:383–390. - PubMed
-
- Borgius L, Restrepo CE, Leao RN, Saleh N, Kiehn O. A transgenic mouse line for molecular genetic analysis of excitatory glutamatergic neurons. Mol Cell Neurosci. 2010;45:245–257. - PubMed
-
- Cameron DL, Wessendorf MW, Williams JT. A subset of ventral tegmental area neurons is inhibited by dopamine, 5-hydroxytryptamine and opioids. Neuroscience. 1997;77:155–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
