Loss of interleukin receptor-associated kinase 4 signaling suppresses amyloid pathology and alters microglial phenotype in a mouse model of Alzheimer's disease
- PMID: 23100432
- PMCID: PMC3505880
- DOI: 10.1523/JNEUROSCI.1729-12.2012
Loss of interleukin receptor-associated kinase 4 signaling suppresses amyloid pathology and alters microglial phenotype in a mouse model of Alzheimer's disease
Abstract
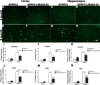
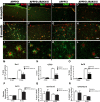
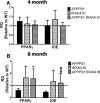
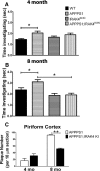
Alzheimer's disease (AD) is typified by the deposition of amyloid in the brain, which elicits a robust microglial-mediated inflammatory response that is associated with disease exacerbation and accelerated progression. Microglia are the principal immune effector cells in the brain and interact with fibrillar forms of Aβ (fAβ) through a receptor complex that includes Toll-like receptors (TLR) 2/4/6 and their coreceptors. Interleukin receptor-associated kinases (IRAKs) are essential intracellular signaling molecules for transduction of TLR signals. Studies of mouse models of AD in which the individual TLRs are knocked out have produced conflicting results on roles of TLR signaling in amyloid homeostasis. Therefore, we disrupted a common downstream TLR signaling element, IRAK4. We report that microglial IRAK4 is necessary in vitro for fAβ to activate the canonical pro-inflammatory signaling pathways leading to activation of p38, JNK, and ERK MAP kinases and to generate reactive oxygen species. In vivo the loss of IRAK4 function results in decreased Aβ levels in a murine model of AD. This was associated with diminished microgliosis and astrogliosis in aged mice. Analysis of microglia isolated from the adult mouse brain revealed an altered pattern of gene expression associated with changes in microglial phenotype that were associated with expression of IRF transcription factors that govern microglial phenotype. Further, loss of IRAK4 function also promoted amyloid clearance mechanisms, including elevated expression of insulin-degrading enzyme. Finally, blocking IRAK function restored olfactory behavior. These data demonstrate that IRAK4 activation acts normally to regulate microglial activation status and influence amyloid homeostasis in the brain.
Figures




References
-
- An H, Hou J, Zhou J, Zhao W, Xu H, Zheng Y, Yu Y, Liu S, Cao X. Phosphatase SHP-1 promotes TLR- and RIG-I-activated production of type I interferon by inhibiting the kinase IRAK1. Nat Immunol. 2008;9:542–550. - PubMed
-
- Anderson CF, Mosser DM. A novel phenotype for an activated macrophage: the type 2 activated macrophage. J Leukoc Biol. 2002;72:101–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
