Differences in sensitivity to neural timing among cortical areas
- PMID: 23100435
- PMCID: PMC3506386
- DOI: 10.1523/JNEUROSCI.1411-12.2012
Differences in sensitivity to neural timing among cortical areas
Abstract
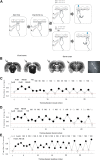
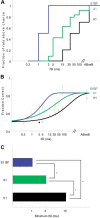
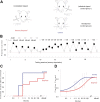
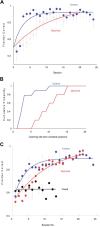
The basic circuitry of auditory, visual, somatosensory and other cortical areas is highly stereotyped (Douglas and Martin, 2004). However, it remains unclear whether this anatomical stereotypy implies functional homogeneity, or whether instead different cortical areas are specialized to process the diverse sensory inputs they receive. Here we have used a two alternative forced choice task to assess modality-specific differences in the ability of rats to exploit precise neuronal timing. We delivered pairs of electrical pulses directly to different areas of cortex to determine the minimum timing differences subjects could detect. By stimulating the cortex directly, we isolated differences due to cortical circuitry rather than to sensory transduction and subcortical processing. Surprisingly, the minimum detectable timing differences varied over more than an order of magnitude, ranging from 1 ms in barrel cortex to 15 ms in visual cortex. Furthermore, these modality-specific differences depended upon sensory experience: although animals subjected to whisker clipping initially showed an impaired ability to exploit fine timing in barrel cortical stimulation, behavioral training partially rescued this deficit. Our results suggest that different cortical areas are adapted to the specific structure of the input signals they process, and that precise spike timing may play a more important role for some cortical areas than for others.
Figures
Similar articles
-
Millisecond-scale differences in neural activity in auditory cortex can drive decisions.Nat Neurosci. 2008 Nov;11(11):1262-3. doi: 10.1038/nn.2211. Epub 2008 Oct 12. Nat Neurosci. 2008. PMID: 18849984 Free PMC article.
-
Experience-Dependent Development of Feature-Selective Synchronization in the Primary Visual Cortex.J Neurosci. 2018 Sep 5;38(36):7852-7869. doi: 10.1523/JNEUROSCI.0027-18.2018. Epub 2018 Jul 31. J Neurosci. 2018. PMID: 30064994 Free PMC article.
-
Intracortical processes regulating the integration of sensory information.Prog Brain Res. 1990;86:129-41. doi: 10.1016/s0079-6123(08)63172-6. Prog Brain Res. 1990. PMID: 1982365 Review.
-
Cross-sensory modulation of primary sensory cortex is developmentally regulated by early sensory experience.J Neurosci. 2011 Feb 16;31(7):2526-36. doi: 10.1523/JNEUROSCI.5547-10.2011. J Neurosci. 2011. PMID: 21325520 Free PMC article.
-
Enriched and deprived sensory experience induces structural changes and rewires connectivity during the postnatal development of the brain.Neural Plast. 2012;2012:305693. doi: 10.1155/2012/305693. Epub 2012 Jul 9. Neural Plast. 2012. PMID: 22848849 Free PMC article. Review.
Cited by
-
History-dependent variability in population dynamics during evidence accumulation in cortex.Nat Neurosci. 2016 Dec;19(12):1672-1681. doi: 10.1038/nn.4403. Epub 2016 Oct 3. Nat Neurosci. 2016. PMID: 27694990 Free PMC article.
-
Learning in a sensory cortical microstimulation task is associated with elevated representational stability.Nat Commun. 2023 Jun 29;14(1):3860. doi: 10.1038/s41467-023-39542-x. Nat Commun. 2023. PMID: 37385989 Free PMC article.
-
Synaptic plasticity as a cortical coding scheme.Curr Opin Neurobiol. 2015 Dec;35:185-99. doi: 10.1016/j.conb.2015.10.003. Epub 2015 Nov 3. Curr Opin Neurobiol. 2015. PMID: 26497430 Free PMC article. Review.
-
Cortical Neuroprosthesis Merges Visible and Invisible Light Without Impairing Native Sensory Function.eNeuro. 2017 Dec 20;4(6):ENEURO.0262-17.2017. doi: 10.1523/ENEURO.0262-17.2017. eCollection 2017 Nov-Dec. eNeuro. 2017. PMID: 29279860 Free PMC article.
-
Transsynaptic modality codes in the brain: possible involvement of synchronized spike timing, microRNAs, exosomes and epigenetic processes.Front Integr Neurosci. 2013 Jan 4;6:126. doi: 10.3389/fnint.2012.00126. eCollection 2012. Front Integr Neurosci. 2013. PMID: 23316146 Free PMC article.
References
-
- Ahissar E, Arieli A. Figuring space by time. Neuron. 2001;32:185–201. - PubMed
-
- Bair W, Koch C. Temporal precision of spike trains in extrastriate cortex of the behaving macaque monkey. Neural Comput. 1996;8:1185–1202. - PubMed
-
- Brand A, Behrend O, Marquardt T, McAlpine D, Grothe B. Precise inhibition is essential for microsecond interaural time difference coding. Nature. 2002;417:543–547. - PubMed
-
- Buracas GT, Zador AM, DeWeese MR, Albright TD. Efficient discrimination of temporal patterns by motion-sensitive neurons in primate visual cortex. Neuron. 1998;20:959–969. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
