Emergence of selectivity and tolerance in the avian auditory cortex
- PMID: 23100437
- PMCID: PMC3498467
- DOI: 10.1523/JNEUROSCI.0845-12.2012
Emergence of selectivity and tolerance in the avian auditory cortex
Abstract
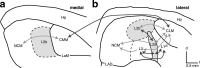
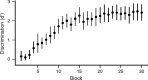
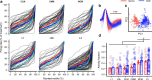
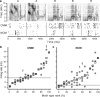
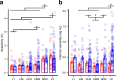
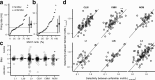
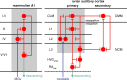
The ability to recognize auditory objects like words and bird songs is thought to depend on neural responses that are selective between categories of the objects and tolerant of variation within those categories. To determine whether a hierarchy of increasing selectivity and tolerance exists in the avian auditory system, we trained European starlings (Sturnus vulgaris) to differentially recognize sets of songs, then measured extracellular single unit responses under urethane anesthesia in six areas of the auditory cortex. Responses were analyzed with a novel, generalized linear mixed model that provides robust estimates of the variance in responses to different stimuli. There were significant differences between areas in selectivity, tolerance, and the effects of training. The L2b and L1 subdivisions of field L had the least selectivity and tolerance. The caudal nidopallium (NCM) and subdivision L3 of field L were more selective than other areas, whereas the medial and lateral caudal mesopallium were more tolerant than NCM or L2b. L3 had a multimodal distribution of tolerance. Sensitivity to songs that were familiar and those that were not also distinguished the responses of caudomedial mesopallium and NCM. There were significant differences across areas between neurons with wide and narrow spikes. Collectively these results do not fit the traditional hierarchical view of the avian auditory forebrain, but are consistent with emerging concepts homologizing avian cortical and neocortical circuitry. The results suggest a functional divergence within the cortex into processing streams that respond to complementary aspects of the variability in communicative sounds.
Figures


References
-
- Adret-Hausberger M, Jenkins P. Complex organization of the warbling song in starlings. Behaviour. 1988;107:138–156.
-
- Bonke D, Scheich H, Langner G. Responsiveness of units in the auditory neostriatum of the guinea fowl (Numida meleagris) to species-specific calls and synthetic stimuli. I. Tonotopy and functional zones. J Comp Physiol. 1979;132:243–255.
-
- Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7:434–455.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
