Apolipoprotein E, especially apolipoprotein E4, increases the oligomerization of amyloid β peptide
- PMID: 23100439
- PMCID: PMC3493562
- DOI: 10.1523/JNEUROSCI.1542-12.2012
Apolipoprotein E, especially apolipoprotein E4, increases the oligomerization of amyloid β peptide
Abstract
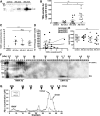
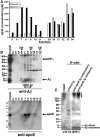
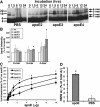
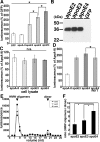
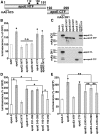
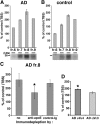
Alzheimer's disease (AD) is the most common progressive neurodegenerative disorder causing dementia. Massive deposition of amyloid β peptide (Aβ) as senile plaques in the brain is the pathological hallmark of AD, but oligomeric, soluble forms of Aβ have been implicated as the synaptotoxic component. The apolipoprotein E ε 4 (apoE ε4) allele is known to be a genetic risk factor for developing AD. However, it is still unknown how apoE impacts the process of Aβ oligomerization. Here, we found that the level of Aβ oligomers in APOE ε4/ε4 AD patient brains is 2.7 times higher than those in APOE ε3/ε3 AD patient brains, matched for total plaque burden, suggesting that apoE4 impacts the metabolism of Aβ oligomers. To test this hypothesis, we examined the effect of apoE on Aβ oligomer formation. Using both synthetic Aβ and a split-luciferase method for monitoring Aβ oligomers, we observed that apoE increased the level of Aβ oligomers in an isoform-dependent manner (E2 < E3 < E4). This effect appears to be dependent on the ApoE C-terminal domain. Moreover, these results were confirmed using endogenous apoE isolated from the TBS-soluble fraction of human brain, which increased the formation of Aβ oligomers. Together, these data show that lipidated apoE, especially apoE4, increases Aβ oligomers in the brain. Higher levels of Aβ oligomers in the brains of APOE ε4/ε4 carriers compared with APOE ε3/ε3 carriers may increase the loss of dendritic spines and accelerate memory impairments, leading to earlier cognitive decline in AD.
Figures

References
-
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
