Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis
- PMID: 23100531
- PMCID: PMC3503234
- DOI: 10.1073/pnas.1119964109
Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis
Abstract
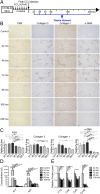
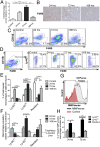
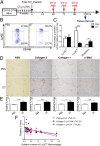
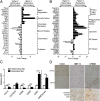
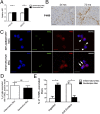
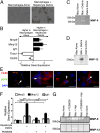
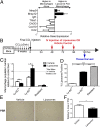
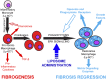
Although macrophages are widely recognized to have a profibrotic role in inflammation, we have used a highly tractable CCl(4)-induced model of reversible hepatic fibrosis to identify and characterize the macrophage phenotype responsible for tissue remodeling: the hitherto elusive restorative macrophage. This CD11B(hi) F4/80(int) Ly-6C(lo) macrophage subset was most abundant in livers during maximal fibrosis resolution and represented the principle matrix metalloproteinase (MMP) -expressing subset. Depletion of this population in CD11B promoter-diphtheria toxin receptor (CD11B-DTR) transgenic mice caused a failure of scar remodeling. Adoptive transfer and in situ labeling experiments showed that these restorative macrophages derive from recruited Ly-6C(hi) monocytes, a common origin with profibrotic Ly-6C(hi) macrophages, indicative of a phenotypic switch in vivo conferring proresolution properties. Microarray profiling of the Ly-6C(lo) subset, compared with Ly-6C(hi) macrophages, showed a phenotype outside the M1/M2 classification, with increased expression of MMPs, growth factors, and phagocytosis-related genes, including Mmp9, Mmp12, insulin-like growth factor 1 (Igf1), and Glycoprotein (transmembrane) nmb (Gpnmb). Confocal microscopy confirmed the postphagocytic nature of restorative macrophages. Furthermore, the restorative macrophage phenotype was recapitulated in vitro by the phagocytosis of cellular debris with associated activation of the ERK signaling cascade. Critically, induced phagocytic behavior in vivo, through administration of liposomes, increased restorative macrophage number and accelerated fibrosis resolution, offering a therapeutic strategy to this orphan pathological process.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Hayden T. Scarred by disease. Nat Med. 2011;17(1):18–20. - PubMed
-
- Ramachandran P, Iredale JP. Reversibility of liver fibrosis. Ann Hepatol. 2009;8(4):283–291. - PubMed
-
- Eddy AA. Can renal fibrosis be reversed? Pediatr Nephrol. 2005;20(10):1369–1375. - PubMed
-
- Pickrell JA, Diel JH, Slauson DO, Halliwell WH, Mauderly JL. Radiation-induced pulmonary fibrosis resolves spontaneously if dense scars are not formed. Exp Mol Pathol. 1983;38(1):22–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

