Association of a NOD2 gene polymorphism and T-helper 17 cells with presumed ocular toxoplasmosis
- PMID: 23100559
- PMCID: PMC3523795
- DOI: 10.1093/infdis/jis640
Association of a NOD2 gene polymorphism and T-helper 17 cells with presumed ocular toxoplasmosis
Abstract
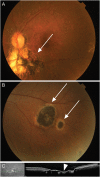
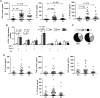
Retinochoroiditis manifests in patients infected with Toxoplasma gondii. Here, we assessed 30 sibships and 89 parent/case trios of presumed ocular toxoplasmosis (POT) to evaluate associations with polymorphisms in the NOD2 gene. Three haplotype-tagging single-nucleotide polymorphisms (tag-SNPs) within the NOD2 gene were genotyped. The family-based association test showed that the tag-SNP rs3135499 is associated with retinochoroiditis (P = .039). We then characterized the cellular immune response of 59 cases of POT and 4 cases of active ocular toxoplasmosis (AOT). We found no differences in levels of interferon γ (IFN-γ) and interleukin 2 produced by T-helper 1 cells when comparing patients with AOT or POT to asymptomatic individuals. Unexpectedly, we found an increased interleukin 17A (IL-17A) production in patients with POT or OAT. In patients with POT or AOT, the main cellular source of IL-17A was CD4(+)CD45RO(+)T-bet(-)IFN-γ(-) T-helper 17 cells. Altogether, our results suggest that NOD2 influences the production of IL-17A by CD4(+) T lymphocytes and might contribute to the development of ocular toxoplasmosis.
Figures



References
-
- Olariu TR, Remington JS, McLeod R, Alam A, Montoya JG. Severe congenital toxoplasmosis in the United States: clinical and serologic findings in untreated infants. Pediatr Infect Dis J. 2011;30:1056–61. - PubMed
-
- Holland GN. Ocular toxoplasmosis: a global reassessment. Part II: disease manifestations and management. Am J Ophthalmol. 2004;137:1–17. - PubMed
-
- Portela RW, Bethony J, Costa MI, et al. A multihousehold study reveals a positive correlation between age, severity of ocular toxoplasmosis, and levels of glycoinositolphospholipid-specific immunoglobulin A. J Infect Dis. 2004;190:175–83. - PubMed
-
- Glasner PD, Silveira C, Kruszon-Moran D, et al. An unusually high prevalence of ocular toxoplasmosis in southern Brazil. Am J Ophthalmol. 1992;114:136–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

