Rationale and design of the RIACT-study: a multi-center placebo controlled double blind study to test the efficacy of RItuximab in Acute Cellular tubulointerstitial rejection with B-cell infiltrates in renal Transplant patients: study protocol for a randomized controlled trial
- PMID: 23101480
- PMCID: PMC3522060
- DOI: 10.1186/1745-6215-13-199
Rationale and design of the RIACT-study: a multi-center placebo controlled double blind study to test the efficacy of RItuximab in Acute Cellular tubulointerstitial rejection with B-cell infiltrates in renal Transplant patients: study protocol for a randomized controlled trial
Abstract
Background: Acute kidney allograft rejection is a major cause for declining graft function and has a negative impact on the long-term graft survival. The majority (90%) of acute rejections are T-cell mediated and, therefore, the anti-rejection therapy targets T-cell-mediated mechanisms of the rejection process. However, there is increasing evidence that intragraft B-cells are also important in the T-cell-mediated rejections. First, a significant proportion of patients with acute T-cell-mediated rejection have B-cells present in the infiltrates. Second, the outcome of these patients is inferior, which has been related to an inferior response to the conventional anti-rejection therapy. Third, treatment of these patients with an anti-CD20 antibody (rituximab) improves the allograft outcome as reported in single case observations and in one small study. Despite the promise of these observations, solid evidence is required before incorporating this treatment option into a general treatment recommendation.
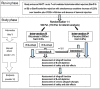
Methods/design: The RIACT study is designed as a randomized, double-blind, placebo-controlled, parallel group multicenter Phase III study. The study examines whether rituximab, in addition to the standard treatment with steroid-boli, leads to an improved one-year kidney allograft function, compared to the standard treatment alone in patients with acute T-cell mediated tubulointerstitial rejection and significant B-cell infiltrates in their biopsies. A total of 180 patients will be recruited.
Discussion: It is important to clarify the relevance of anti-B cell targeting in T-cell mediated rejection and answer the question whether this novel concept should be incorporated in the conventional anti-rejection therapy.
Trial registration: Clinical trials gov. number: NCT01117662.
Figures
References
-
- KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–S155. - PubMed
-
- Webster A, Pankhurst T, Rinaldi F, Chapman JR, Craig JC. Polyclonal and monoclonal antibodies for treating acute rejection episodes in kidney transplant recipients. Cochrane Database Syst Rev. 2006;2:CD004756. - PubMed
-
- Massy ZA, Guijarro C, Kasiske BL. Clinical predictors of chronic renal allograft rejection. Kidney Int Suppl. 1995;52:S85–S88. - PubMed
-
- Nickerson P, Jeffery J, Gough J, McKenna R, Grimm P, Cheang M, Rush D. Identification of clinical and histopathologic risk factors for diminished renal function 2 years posttransplant. J Am Soc Nephrol. 1998;9:482–487. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical