A mouse model of Salmonella typhi infection
- PMID: 23101627
- PMCID: PMC3500584
- DOI: 10.1016/j.cell.2012.08.042
A mouse model of Salmonella typhi infection
Abstract
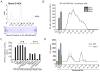
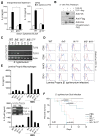
Salmonella spp. are gram-negative flagellated bacteria that can cause food- and waterborne gastroenteritis and typhoid fever in humans. We now report that flagellin from Salmonella spp. is recognized in mouse intestine by Toll-like receptor 11 (TLR11). Absence of TLR11 renders mice more susceptible to infection by S. Typhimurium, with increased dissemination of the bacteria and enhanced lethality. Unlike S. Typhimurium, S. Typhi, a human obligatory pathogen that causes typhoid fever, is normally unable to infect mice. TLR11 is expressed in mice, but not in humans, and remarkably, we find that tlr11(-/-) mice are efficiently infected with orally administered S. Typhi. We also find that tlr11(-/-) mice can be immunized against S. Typhi. Therefore, tlr11(-/-) mice represent a small-animal model for the study of the immune response to S. Typhi and for the development of vaccines against this important human pathogen.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




Comment in
-
A tollgate for typhoid.Cell. 2012 Oct 26;151(3):473-5. doi: 10.1016/j.cell.2012.10.016. Cell. 2012. PMID: 23101620 Free PMC article.
-
Absence of TLR11 in Mice Does Not Confer Susceptibility to Salmonella Typhi.Cell. 2016 Feb 25;164(5):827-8. doi: 10.1016/j.cell.2016.02.015. Cell. 2016. PMID: 26919416 Free PMC article. No abstract available.
-
Mice Lacking TLR11 Exhibit Variable Salmonella typhi Susceptibility.Cell. 2016 Feb 25;164(5):829-30. doi: 10.1016/j.cell.2016.02.020. Cell. 2016. PMID: 26919417 No abstract available.
References
-
- Arricau N, Hermant D, Waxin H, Ecobichon C, Duffey PS, Popoff MY. The RcsB-RcsC regulatory system of Salmonella typhi differentially modulates the expression of invasion proteins, flagellin and Vi antigen in response to osmolarity. Mol Microbiol. 1998;29:835–850. - PubMed
-
- Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

