Gross lesions of alimentary disease in adult cattle
- PMID: 23101672
- PMCID: PMC7125524
- DOI: 10.1016/j.cvfa.2012.07.009
Gross lesions of alimentary disease in adult cattle
Abstract
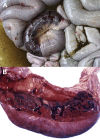
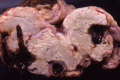
The purpose of the gross necropsy examination of the gastrointestinal tract is to recognize the presence of lesions, thus requiring a basic understanding of its normal appearance and anatomy. This article highlights gross changes to the gastrointestinal tract of adult cattle that help place the disease processes into broad categories. Although few gross lesions reach the zenith of pathognomonic, there are numerous lesions that, when considered in aggregate with history (eg, number of animals affected, environment, duration of signs, time of onset relative to management changes, previous management) and clinical signs, can help narrow the spectrum of causes, provide a basis for a strong presumptive diagnosis, and focus diagnostic test selection.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


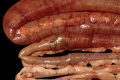

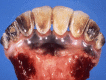
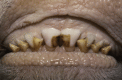


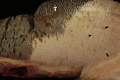

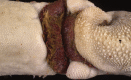
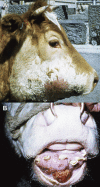

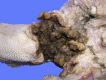
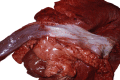
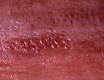
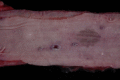

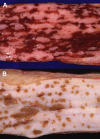
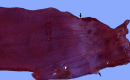

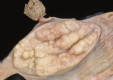






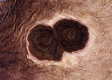
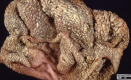
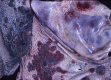



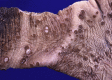
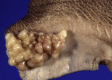
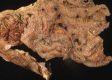

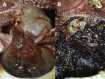
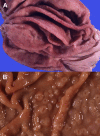
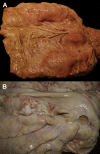
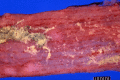
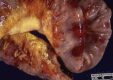
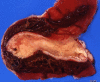
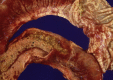
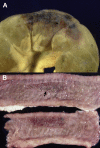

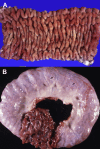
Similar articles
-
Ultrasonography of the gastrointestinal tract in cattle.Vet Clin North Am Food Anim Pract. 2009 Nov;25(3):567-90, Table of Contents. doi: 10.1016/j.cvfa.2009.07.004. Vet Clin North Am Food Anim Pract. 2009. PMID: 19825434 Review.
-
Lesions of bovine ruminal tympany.J Am Vet Med Assoc. 1970 Oct 1;157(7):947-52. J Am Vet Med Assoc. 1970. PMID: 5472344 No abstract available.
-
Gastrointestinal Nematodes, Diagnosis and Control.Vet Clin North Am Food Anim Pract. 2018 Mar;34(1):185-199. doi: 10.1016/j.cvfa.2017.10.008. Vet Clin North Am Food Anim Pract. 2018. PMID: 29421029 Review.
-
Diagnostic value of tissue biopsy in gastrointestinal and liver disease.Vet Rec. 1987 Mar 7;120(10):230-3. doi: 10.1136/vr.120.10.230. Vet Rec. 1987. PMID: 3554717 Review.
-
Field necropsy of cattle and diagnostic sample submission.Vet Clin North Am Food Anim Pract. 2012 Nov;28(3):391-405. doi: 10.1016/j.cvfa.2012.07.006. Epub 2012 Aug 30. Vet Clin North Am Food Anim Pract. 2012. PMID: 23101667 Review.
Cited by
-
Bovine trichomoniasis: A hidden threat to reproductive efficiency.Open Vet J. 2024 Nov;14(11):2722-2730. doi: 10.5455/OVJ.2024.v14.i11.1. Epub 2024 Nov 30. Open Vet J. 2024. PMID: 39737020 Free PMC article. Review.
References
-
- Schummer A., Nickel R., Sack W.O. The viscera of the domestic mammals. 2nd revised edition. Springer-Verlag; New York: 1979. The alimentary canal of the ruminants; p. 148. 168.
-
- Dyce K.M., Sack W.O., Wensing C.J. Textbook of veterinary anatomy. 3rd edition. Saunders; Philadelphia: 2002. The head and ventral neck of the ruminants; pp. 636–637.
-
- Helman R.G. Interpretation of basic gross pathologic changes of the digestive tract. Vet Clin North Am Food Anim Pract. 2000;16:1–22. - PubMed
-
- Thompson K. Bones and joints. In: Maxie M.G., editor. 5th edition. vol. 1. Elsevier; 2007. pp. 98–99. (Jubb, Kennedy, and Palmer’s Pathology of domestic animals).
-
- Brown C.C., Baker D.C., Barker I.K. Alimentary System. In: Maxie M.G., editor. 5th edition. vol. 2. Elsevier; 2007. pp. 20–21. (Jubb, Kennedy, and Palmer’s Pathology of domestic animals).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

