Synthesis of the C1-C26 hexacyclic subunit of pectenotoxin 2
- PMID: 23101680
- PMCID: PMC3500424
- DOI: 10.1021/ol302751b
Synthesis of the C1-C26 hexacyclic subunit of pectenotoxin 2
Abstract
Synthesis of the C1-C26 hexacyclic subunit of pectenotoxin-2 (PTX-2) is described that features a stereoselective annulation to generate the C-ring by triple asymmetric Nozaki-Hiyama-Kishi coupling followed by oxidative cyclization. Preparation of the C1-C14 AB spriroketal-containing subunit employs a recently developed metallacycle-mediated reductive cross-coupling between a TMS-alkyne and a terminal alkene.
Figures

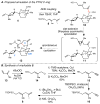



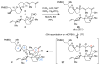

References
-
- Chae H, Choi T, Kim B, Jung J, Bang Y, Shin DY. Oncogene. 2005;24:4813–4819. - PubMed
-
- Jung JH, Sim CJ, Lee C. J Nat Prod. 1995;58:1722–1726. - PubMed
-
- Spector I, Braet F, Shochet nR, Bubb MR. Micro Res And Tech. 1999;47:18–37. - PubMed
-
- Yasumoto T, Murate M, Oshima Y, Sano M, Matsumoto GK, Clardy J. Tetrahedron. 1985;41:1019–1025.
- Sasaki K, Wright JLC, Yasumoto T. J Org Chem. 1998;63:2475–2480. - PubMed
- Yasumoto T, Murata M. Chem Rev. 1993;93:1897–1909.
-
- Yasumoto T, Oshima Y, Sugawara W, Fukuyo Y, Oguri H, Igarashi T, Fujita N. Bull Jpn Soc Sci Fish. 1980;46:1405–1411.
- Lee J, Igarashi T, Fraga S, Dahl E, Hovgaard P, Yasumoto T. J Appl Phycol. 1989;1:147–152.
- Draisci R, Lucentini L, Giannetti L, Boria P, Poletti R. Toxicon. 1996;34:923–935. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

