Gadd45γ and Map3k4 interactions regulate mouse testis determination via p38 MAPK-mediated control of Sry expression
- PMID: 23102580
- PMCID: PMC3526779
- DOI: 10.1016/j.devcel.2012.09.016
Gadd45γ and Map3k4 interactions regulate mouse testis determination via p38 MAPK-mediated control of Sry expression
Abstract
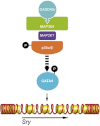
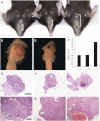
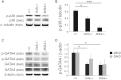
Loss of the kinase MAP3K4 causes mouse embryonic gonadal sex reversal due to reduced expression of the testis-determining gene, Sry. However, because of widespread expression of MAP3K4, the cellular basis of this misregulation was unclear. Here, we show that mice lacking Gadd45γ also exhibit XY gonadal sex reversal caused by disruption to Sry expression. Gadd45γ is expressed in a dynamic fashion in somatic cells of the developing gonads from 10.5 days postcoitum (dpc) to 12.5 dpc. Gadd45γ and Map3k4 genetically interact during sex determination, and transgenic overexpression of Map3k4 rescues gonadal defects in Gadd45γ-deficient embryos. Sex reversal in both mutants is associated with reduced phosphorylation of p38 MAPK and GATA4. In addition, embryos lacking both p38α and p38β also exhibit XY gonadal sex reversal. Taken together, our data suggest a requirement for GADD45γ in promoting MAP3K4-mediated activation of p38 MAPK signaling in embryonic gonadal somatic cells for testis determination in the mouse.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures





References
-
- Adams R.H., Porras A., Alonso G., Jones M., Vintersten K., Panelli S., Valladares A., Perez L., Klein R., Nebreda A.R. Essential role of p38alpha MAP kinase in placental but not embryonic cardiovascular development. Mol. Cell. 2000;6:109–116. - PubMed
-
- Albrecht K.H., Eicher E.M. Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev. Biol. 2001;240:92–107. - PubMed
-
- Barreto G., Schäfer A., Marhold J., Stach D., Swaminathan S.K., Handa V., Döderlein G., Maltry N., Wu W., Lyko F., Niehrs C. Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature. 2007;445:671–675. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

