Cardiovascular protection by ApoE and ApoE-HDL linked to suppression of ECM gene expression and arterial stiffening
- PMID: 23103162
- PMCID: PMC3535179
- DOI: 10.1016/j.celrep.2012.09.018
Cardiovascular protection by ApoE and ApoE-HDL linked to suppression of ECM gene expression and arterial stiffening
Abstract
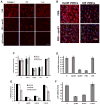
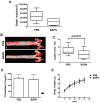
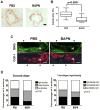
Arterial stiffening is a risk factor for cardiovascular disease, but how arteries stay supple is unknown. Here, we show that apolipoprotein E (apoE) and apoE-containing high-density lipoprotein (apoE-HDL) maintain arterial elasticity by suppressing the expression of extracellular matrix genes. ApoE interrupts a mechanically driven feed-forward loop that increases the expression of collagen-I, fibronectin, and lysyl oxidase in response to substratum stiffening. These effects are independent of the apoE lipid-binding domain and transduced by Cox2 and miR-145. Arterial stiffness is increased in apoE null mice. This stiffening can be reduced by administration of the lysyl oxidase inhibitor BAPN, and BAPN treatment attenuates atherosclerosis despite highly elevated cholesterol. Macrophage abundance in lesions is reduced by BAPN in vivo, and monocyte/macrophage adhesion is reduced by substratum softening in vitro. We conclude that apoE and apoE-containing HDL promote healthy arterial biomechanics and that this confers protection from cardiovascular disease independent of the established apoE-HDL effect on cholesterol.
Copyright © 2012 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Ali ZA, Alp NJ, Lupton H, Arnold N, Bannister T, Hu Y, Mussa S, Wheatcroft M, Greaves DR, Gunn J, et al. Increased in-stent stenosis in ApoE knockout mice: insights from a novel mouse model of balloon angioplasty and stenting. Arterioscler Thromb Vasc Biol. 2007;27:833–840. - PubMed
-
- Bellien J, Thuillez C, Joannides R. Contribution of endothelium-derived hyperpolarizing factors to the regulation of vascular tone in humans. Fundam Clin Pharmacol. 2008;22:363–377. - PubMed
-
- Berry CL, Greenwald SE, Menahem N. Effect of beta-aminopropionitrile on the static elastic properties and blood pressure of spontaneously hypertensive rats. Cardiovascular research. 1981;15:373–381. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

