The transporter Spns2 is required for secretion of lymph but not plasma sphingosine-1-phosphate
- PMID: 23103166
- PMCID: PMC3616498
- DOI: 10.1016/j.celrep.2012.09.021
The transporter Spns2 is required for secretion of lymph but not plasma sphingosine-1-phosphate
Abstract
Plasma sphingosine-1-phosphate (S1P) regulates vascular permeability, and plasma and lymph S1P guide lymphocyte egress from lymphoid organs. S1P is made intracellularly, and little is known about how S1P is delivered into circulatory fluids. Here, we find that mice without the major facilitator superfamily transporter Spns2 have a profound reduction in lymph S1P, but only a minor decrease in plasma S1P. Spns2-deficient mice have a redistribution of lymphocytes from the spleen to lymph nodes and a loss of circulating lymphocytes, consistent with normal egress from the spleen directed by plasma S1P and blocked egress from lymph nodes directed by lymph S1P. Spns2 is needed in endothelial cells to supply lymph S1P and support lymphocyte circulation. As a differential requirement for lymph and blood S1P, Spns2 may be an attractive target for immune suppressive drugs.
Copyright © 2012 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

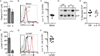

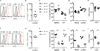
References
-
- Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, Aradhye S, Burtin P. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9:883–897. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

