Distinct single-cell morphological dynamics under beta-lactam antibiotics
- PMID: 23103254
- PMCID: PMC3525771
- DOI: 10.1016/j.molcel.2012.09.016
Distinct single-cell morphological dynamics under beta-lactam antibiotics
Abstract
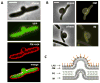
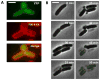
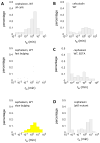
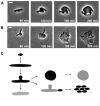
The bacterial cell wall is conserved in prokaryotes, stabilizing cells against osmotic stress. Beta-lactams inhibit cell-wall synthesis and induce lysis through a bulge-mediated mechanism; however, little is known about the formation dynamics and stability of these bulges. To capture processes of different timescales, we developed an imaging platform combining automated image analysis with live-cell microscopy at high time resolution. Beta-lactam killing of Escherichia coli cells proceeded through four stages: elongation, bulge formation, bulge stagnation, and lysis. Both the cell wall and outer membrane (OM) affect the observed dynamics; damaging the cell wall with different beta-lactams and compromising OM integrity cause different modes and rates of lysis. Our results show that the bulge-formation dynamics are determined by how the cell wall is perturbed. The OM plays an independent role in stabilizing the bulge once it is formed. The stabilized bulge delays lysis and allows recovery upon drug removal.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


References
-
- Daly KE, Huang KC, Wingreen NS, Mukhopadhyay R. Mechanics of membrane bulging during cell-wall disruption in Gram-negative bacteria. Phys Rev E Stat Nonlin Soft Matter Phys. 2011;83:041922. - PubMed
-
- de Pedro MA, Holtje JV, Schwarz H. Fast lysis of Escherichia coli filament cells requires differentiation of potential division sites. Microbiology. 2002;148:79–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

