Phosphorus limitation increases attachment in Agrobacterium tumefaciens and reveals a conditional functional redundancy in adhesin biosynthesis
- PMID: 23103488
- PMCID: PMC3656598
- DOI: 10.1016/j.resmic.2012.10.013
Phosphorus limitation increases attachment in Agrobacterium tumefaciens and reveals a conditional functional redundancy in adhesin biosynthesis
Abstract
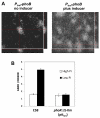
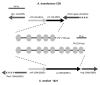
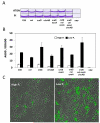
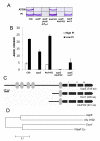
Bacterial responses to phosphorus limitation, commonly inorganic phosphate (P(i)), are important survival mechanisms in a variety of environments. The two-component sensor kinase PhoR and its cognate response regulator PhoB are central to the P(i) limitation response of many bacteria and control the large Pho regulon. Limitation for P(i) significantly increased attachment and biofilm formation by the plant pathogen Agrobacterium tumefaciens, and this was driven by PhoB. Surprisingly, it was also found that both phoR and phoB were essential in A. tumefaciens. Expression of a plasmid-borne copy of the low affinity P(i) transporter (pit) from Sinorhizobium meliloti in A. tumefaciens abolished the phoB and phoR essentiality in A. tumefaciens and allowed direct demonstration of the requirement for this regulatory system in the biofilm response. Increased attachment under P(i) limitation required a unipolar polysaccharide (UPP) adhesin. Mutation of a polyisoprenylphosphate hexose-1-phosphate transferase (PHPT) called uppE abolished UPP production and prevented surface attachment under P(i)-replete conditions, but this was rescued under P(i) limitation, and this rescue required phoB. In low P(i) conditions, either uppE or a paralogous gene Atu0102 is functionally redundant, but only uppE functions in UPP synthesis and attachment when P(i) is replete. This conditional functional redundancy illustrates the influence of phosphorus availability on A. tumefaciens surface colonization.
Copyright © 2012 Institut Pasteur. Published by Elsevier Masson SAS. All rights reserved.
Figures

Similar articles
-
Phosphorus limitation enhances biofilm formation of the plant pathogen Agrobacterium tumefaciens through the PhoR-PhoB regulatory system.J Bacteriol. 2004 Jul;186(14):4492-501. doi: 10.1128/JB.186.14.4492-4501.2004. J Bacteriol. 2004. PMID: 15231781 Free PMC article.
-
Genetic analysis of Agrobacterium tumefaciens unipolar polysaccharide production reveals complex integrated control of the motile-to-sessile switch.Mol Microbiol. 2013 Sep;89(5):929-48. doi: 10.1111/mmi.12321. Epub 2013 Jul 29. Mol Microbiol. 2013. PMID: 23829710 Free PMC article.
-
Discrete Responses to Limitation for Iron and Manganese in Agrobacterium tumefaciens: Influence on Attachment and Biofilm Formation.J Bacteriol. 2015 Dec 28;198(5):816-29. doi: 10.1128/JB.00668-15. J Bacteriol. 2015. PMID: 26712936 Free PMC article.
-
Function and Regulation of Agrobacterium tumefaciens Cell Surface Structures that Promote Attachment.Curr Top Microbiol Immunol. 2018;418:143-184. doi: 10.1007/82_2018_96. Curr Top Microbiol Immunol. 2018. PMID: 29998422 Free PMC article. Review.
-
Mechanisms and regulation of polar surface attachment in Agrobacterium tumefaciens.Curr Opin Microbiol. 2009 Dec;12(6):708-14. doi: 10.1016/j.mib.2009.09.014. Epub 2009 Oct 29. Curr Opin Microbiol. 2009. PMID: 19879182 Free PMC article. Review.
Cited by
-
The Ctp type IVb pilus locus of Agrobacterium tumefaciens directs formation of the common pili and contributes to reversible surface attachment.J Bacteriol. 2014 Aug 15;196(16):2979-88. doi: 10.1128/JB.01670-14. Epub 2014 Jun 9. J Bacteriol. 2014. PMID: 24914181 Free PMC article.
-
Establishing a Role for Bacterial Cellulose in Environmental Interactions: Lessons Learned from Diverse Biofilm-Producing Proteobacteria.Front Microbiol. 2015 Nov 17;6:1282. doi: 10.3389/fmicb.2015.01282. eCollection 2015. Front Microbiol. 2015. PMID: 26635751 Free PMC article. Review.
-
Bacterial biofilms as an essential component of rhizosphere plant-microbe interactions.Methods Microbiol. 2023;53:3-48. doi: 10.1016/bs.mim.2023.05.006. Epub 2023 Jun 22. Methods Microbiol. 2023. PMID: 38415193 Free PMC article. No abstract available.
-
Suppression of Alternative Lipooligosaccharide Glycosyltransferase Activity by UDP-Galactose Epimerase Enhances Murine Lung Infection and Evasion of Serum IgM.Front Cell Infect Microbiol. 2019 May 15;9:160. doi: 10.3389/fcimb.2019.00160. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31157175 Free PMC article.
-
Deciphering bacterial mechanisms of root colonization.Environ Microbiol Rep. 2021 Aug;13(4):428-444. doi: 10.1111/1758-2229.12934. Epub 2021 Feb 15. Environ Microbiol Rep. 2021. PMID: 33538402 Free PMC article. Review.
References
-
- Benning C, Huang Z-H, Gage DA. Accumulation of a novel glycolipid and a betaine lipid in the cells of Rhodobacter sphaeroides grown under phosphate limitation. Arch. Biochem. Biophys. 1995;317:103–111. - PubMed
-
- Chiang SL, Rubin EJ. Construction of a mariner-based transposon for epitope-tagging and genomic targeting. Gene. 2002;296:179–85. - PubMed
-
- Christensen BB, Sternberg C, Andersen JB, Palmer RJJ, Nielsen AT, Givskov M, Molin S. Molecular tools for study of biofilm physiology. Methods Enzymol. 1999;310:20–42. - PubMed
-
- Danhorn T, Fuqua C. Biofilm formation by plant-associated bacteria. Annu. Rev. Microbiol. 2007;61:401–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

