Identification of L- and T-type Ca2+ channels in rat cerebral arteries: role in myogenic tone development
- PMID: 23103495
- PMCID: PMC3543679
- DOI: 10.1152/ajpheart.00476.2012
Identification of L- and T-type Ca2+ channels in rat cerebral arteries: role in myogenic tone development
Abstract
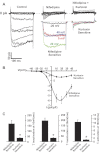
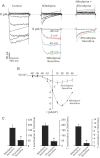
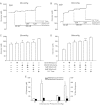
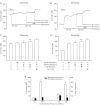
L-type Ca(2+) channels are broadly expressed in arterial smooth muscle cells, and their voltage-dependent properties are important in tone development. Recent studies have noted that these Ca(2+) channels are not singularly expressed in vascular tissue and that other subtypes are likely present. In this study, we ascertained which voltage-gated Ca(2+) channels are expressed in rat cerebral arterial smooth muscle and determined their contribution to the myogenic response. mRNA analysis revealed that the α(1)-subunit of L-type (Ca(v)1.2) and T-type (Ca(v)3.1 and Ca(v)3.2) Ca(2+) channels are present in isolated smooth muscle cells. Western blot analysis subsequently confirmed protein expression in whole arteries. With the use of patch clamp electrophysiology, nifedipine-sensitive and -insensitive Ba(2+) currents were isolated and each were shown to retain electrical characteristics consistent with L- and T-type Ca(2+) channels. The nifedipine-insensitive Ba(2+) current was blocked by mibefradil, kurtoxin, and efonidpine, T-type Ca(2+) channel inhibitors. Pressure myography revealed that L-type Ca(2+) channel inhibition reduced tone at 20 and 80 mmHg, with the greatest effect at high pressure when the vessel is depolarized. In comparison, the effect of T-type Ca(2+) channel blockade on myogenic tone was more limited, with their greatest effect at low pressure where vessels are hyperpolarized. Blood flow modeling revealed that the vasomotor responses induced by T-type Ca(2+) blockade could alter arterial flow by ∼20-50%. Overall, our findings indicate that L- and T-type Ca(2+) channels are expressed in cerebral arterial smooth muscle and can be electrically isolated from one another. Both conductances contribute to myogenic tone, although their overall contribution is unequal.
Figures








References
-
- Ball CJ, Wilson DP, Turner SP, Saint DA, Beltrame JF. Heterogeneity of L- and T-channels in the vasculature: rationale for the efficacy of combined L- and T-blockade. Hypertension 53: 654–660, 2009 - PubMed
-
- Braunstein TH, Inoue R, Cribbs L, Oike M, Ito Y, Holstein-Rathlou NH, Jensen LJ. The role of L- and T-type calcium channels in local and remote calcium responses in rat mesenteric terminal arterioles. J Vasc Res 46: 138–151, 2009 - PubMed
-
- Catterall WA. Structure and function of voltage-gated ion channels. Annu Rev Biochem 64: 493–531, 1995 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

