Ibandronate increases the expression of the pro-apoptotic gene FAS by epigenetic mechanisms in tumor cells
- PMID: 23103563
- PMCID: PMC3557391
- DOI: 10.1016/j.bcp.2012.10.016
Ibandronate increases the expression of the pro-apoptotic gene FAS by epigenetic mechanisms in tumor cells
Abstract
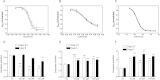
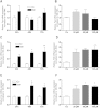
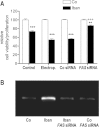
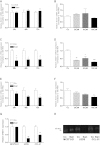
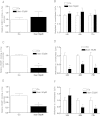
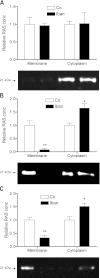
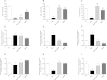
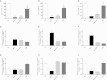
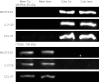
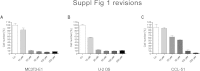
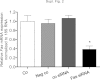
There is growing evidence that aminobisphosphonates like ibandronate show anticancer activity by an unknown mechanism. Biochemically, they prevent posttranslational isoprenylation of small GTPases, thus inhibiting their activity. In tumor cells, activated RAS-GTPase, the founding member of the gene family, down-regulates the expression of the pro-apoptotic gene FAS via epigenetic DNA-methylation by DNMT1. We compared ibandronate treatment in neoplastic human U-2 osteosarcoma and in mouse CCL-51 breast cancer cells as well as in the immortalized non-neoplastic MC3T3-E1 osteoblastic cells. Ibandronate attenuated cell proliferation in all cell lines tested. In the neoplastic cells we found up-regulation of caspases suggesting apoptosis. Further we found stimulation of FAS-expression as a result of epigenetic DNA demethylation that was due to down-regulation of DNMT1, which was rescued by re-isoprenylation by both geranylgeranyl-pyrophosphate and farnesylpyrophosphate. In contrast, ibandronate did not affect FAS and DNMT1 expression in MC3T3-E1 non-neoplastic cells. Data suggest that bisphosphonates via modulation of the activity of small-GTPases induce apoptosis in neoplastic cells by DNA-CpG-demethylation and stimulation of FAS-expression. In conclusion the shown epigenetic mechanism underlying the anti-neoplastic activity of farnesyl-transferase-inhibition, also explains the clinical success of other drugs, which target this pathway.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

References
-
- Caraglia M., Santini D., Marra M., Vincenzi B., Tonini G., Budillon A. Emerging anti-cancer molecular mechanisms of aminobisphosphonates. Endocr Relat Cancer. 2006;13:7–26. - PubMed
-
- Baron R., Ferrari S., Russell R.G. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011;48:677–692. - PubMed
-
- Graaf M.R., Richel D.J., van Noorden C.J., Guchelaar H.J. Effects of statins and farnesyltransferase inhibitors on the development and progression of cancer. Cancer Treat Rev. 2004;30:609–641. - PubMed
-
- Kucukzeybek Y., Gorumlu G., Cengiz E., Karabulut B., Sezgin C., Atmaca H. Apoptosis-mediated cytotoxic effects of ibandronic acid on hormone- and drug-refractory prostate cancer cells and human breast cancer cells. J Int Med Res. 2010;38:1663–1672. - PubMed
-
- Luckman S.P., Hughes D.E., Coxon F.P., Graham R., Russell G., Rogers M.J. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581–589. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

