Biology and therapeutic potential of hydrogen sulfide and hydrogen sulfide-releasing chimeras
- PMID: 23103569
- PMCID: PMC3566320
- DOI: 10.1016/j.bcp.2012.10.019
Biology and therapeutic potential of hydrogen sulfide and hydrogen sulfide-releasing chimeras
Abstract
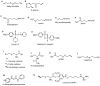
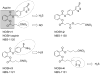
Hydrogen sulfide, H2S, is a colorless gas with a strong odor that until recently was only considered to be a toxic environmental pollutant with little or no physiological significance. However, the past few years have demonstrated its role in many biological systems and it is becoming increasingly clear that H2S is likely to join nitric oxide (NO) and carbon monoxide (CO) as a major player in mammalian biology. In this review, we have provided an overview of the chemistry and biology of H2S and have summarized the chemistry and biological activity of some natural and synthetic H2S-donating compounds. The naturally occurring compounds discussed include, garlic, sulforaphane, erucin, and iberin. The synthetic H2S donors reviewed include, GYY4137; cysteine analogs; S-propyl cysteine, S-allyl cysteine, S-propargyl cysteine, and N-acetyl cysteine. Dithiolethione and its NSAID and other chimeras such as, L-DOPA, sildenafil, aspirin, diclofenac, naproxen, ibuprofen, indomethacin, and mesalamine have also been reviewed in detail. The newly reported NOSH-aspirin that releases both NO and H2S has also been discussed.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

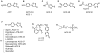

References
-
- Hughes MN, Centelles MN, Moore KP. Making and working with hydrogen sulfide: The chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: a review. Free Radic Biol Med. 2009;47:1346–53. - PubMed
-
- Olson KR. The therapeutic potential of hydrogen sulfide: separating hype from hope. Am J Physiol Regul Integr Comp Physiol. 2011;301:R297–312. - PubMed
-
- Chen KY, Morris JC. Oxidation of sulfide by O2: catalysis and inhibition. Journal of Sanitation Engineering Division. 1972;98:215–27.
-
- DeLeon ER, Stoy GF, Olson KR. Passive loss of hydrogen sulfide in biological experiments. Anal Biochem. 2012;421:203–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

