Test variability of the QuantiFERON-TB gold in-tube assay in clinical practice
- PMID: 23103734
- PMCID: PMC3570654
- DOI: 10.1164/rccm.201203-0430OC
Test variability of the QuantiFERON-TB gold in-tube assay in clinical practice
Abstract
Rationale: Although IFN-γ release assays (IGRAs) are widely used to screen for Mycobacterium tuberculosis infection in high-income countries, published data on repeatability are limited.
Objectives: To determine IGRA repeatability.
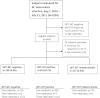
Methods: The study population included consecutive patients referred to The Methodist Hospital (Houston, TX) between August 1, 2010 and July 31, 2011 for latent tuberculosis (TB) infection screening with an IGRA (QuantiFERON-TB Gold In-Tube; Cellestis, Carnegie, Australia). We performed multiple IGRA tests using leftover stimulated plasma according to a prospectively formulated quality control protocol. We analyzed agreement in interpretation of test results classified according to manufacturer-recommended criteria and repeatability of quantitative TB response.
Measurements and main results: During the study period, 1,086 test results were obtained from 543 subjects. Per the manufacturer's cut-point, the result of the second test was discordant from that of the first in 28 (8%) of 366 patients with valid test results, including 13 with an initial negative result and 15 with an initial positive result. Although agreement between repeat test results was high (κ = 0.84; 95% confidence interval, 0.79-0.90), the normal expected range of within-subject variability in TB response on retesting included differences of ± 0.60 IU/ml for all individuals (coefficient of variation, 14%), and ± 0.24 IU/ml (coefficient of variation, 27%) for individuals whose initial TB response was between 0.25 and 0.80 IU/ml.
Conclusions: There is substantial variability in TB response when IGRAs are repeated using the same patient sample. IGRA results should be interpreted cautiously when TB response is near interpretation cut-points.
Figures

Comment in
-
The elusive "gold" standard for detecting Mycobacterium tuberculosis infection.Am J Respir Crit Care Med. 2013 Jan 15;187(2):122-4. doi: 10.1164/rccm.201211-2033ED. Am J Respir Crit Care Med. 2013. PMID: 23322793 No abstract available.
References
-
- Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection–United States, 2010. MMWR Morb Mortal Wkly Rep Recomm Rep 2010;59:1–25 - PubMed
-
- Banoo S, Bell D, Bossuyt P, Herring A, Mabey D, Poole F, Smith PG, Sriram N, Wongsrichanalai C, Linke R, et al. Evaluation of diagnostic tests for infectious diseases: general principles. Nat Rev Microbiol 2006;4:S20–S32 - PubMed
-
- Derion T. Considerations for the planning and conduct of reproducibility studies of in vitro diagnostic tests for infectious agents. Biotechnol Annu Rev 2003;9:249–258 - PubMed
-
- Cellestis. Validation report QuantiFERON-TB Gold In-Tube: reproducibility study. 2006 [accessed 2011 Dec 16]. Available from: http://www.cellestis.com/IRM/Company/ShowPage.aspx/PDFs/1359-10000000/Va....

