Mammalian P4-ATPases and ABC transporters and their role in phospholipid transport
- PMID: 23103747
- PMCID: PMC3562415
- DOI: 10.1016/j.bbalip.2012.10.006
Mammalian P4-ATPases and ABC transporters and their role in phospholipid transport
Abstract
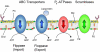
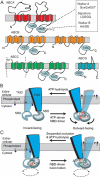
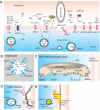
Transport of phospholipids across cell membranes plays a key role in a wide variety of biological processes. These include membrane biosynthesis, generation and maintenance of membrane asymmetry, cell and organelle shape determination, phagocytosis, vesicle trafficking, blood coagulation, lipid homeostasis, regulation of membrane protein function, apoptosis, etc. P(4)-ATPases and ATP binding cassette (ABC) transporters are the two principal classes of membrane proteins that actively transport phospholipids across cellular membranes. P(4)-ATPases utilize the energy from ATP hydrolysis to flip aminophospholipids from the exocytoplasmic (extracellular/lumen) to the cytoplasmic leaflet of cell membranes generating membrane lipid asymmetry and lipid imbalance which can induce membrane curvature. Many ABC transporters play crucial roles in lipid homeostasis by actively transporting phospholipids from the cytoplasmic to the exocytoplasmic leaflet of cell membranes or exporting phospholipids to protein acceptors or micelles. Recent studies indicate that some ABC proteins can also transport phospholipids in the opposite direction. The importance of P(4)-ATPases and ABC transporters is evident from the findings that mutations in many of these transporters are responsible for severe human genetic diseases linked to defective phospholipid transport. This article is part of a Special Issue entitled Phospholipids and Phospholipid Metabolism.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures



References
-
- Holthuis JC, Levine TP. Lipid traffic: floppy drives and a superhighway. Nat Rev Mol Cell Biol. 2005;6:209–220. - PubMed
-
- Daleke DL. Phospholipid flippases. J Biol Chem. 2007;282:821–825. - PubMed
-
- Folmer DE, Elferink RP, Paulusma CC. P4 ATPases - lipid flippases and their role in disease. Biochim Biophys Acta. 2009;1791:628–635. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

