Loss of 53BP1 causes PARP inhibitor resistance in Brca1-mutated mouse mammary tumors
- PMID: 23103855
- PMCID: PMC7518105
- DOI: 10.1158/2159-8290.CD-12-0049
Loss of 53BP1 causes PARP inhibitor resistance in Brca1-mutated mouse mammary tumors
Abstract
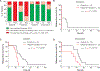
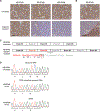
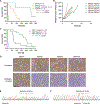
Inhibition of PARP is a promising therapeutic strategy for homologous recombination-deficient tumors, such as BRCA1-associated cancers. We previously reported that BRCA1-deficient mouse mammary tumors may acquire resistance to the clinical PARP inhibitor (PARPi) olaparib through activation of the P-glycoprotein drug efflux transporter. Here, we show that tumor-specific genetic inactivation of P-glycoprotein increases the long-term response of BRCA1-deficient mouse mammary tumors to olaparib, but these tumors eventually developed PARPi resistance. In a fraction of cases, this resistance is caused by partial restoration of homologous recombination due to somatic loss of 53BP1. Importantly, PARPi resistance was minimized by long-term treatment with the novel PARP inhibitor AZD2461, which is a poor P-glycoprotein substrate. Together, our data suggest that restoration of homologous recombination is an important mechanism for PARPi resistance in BRCA1-deficient mammary tumors and that the risk of relapse of BRCA1-deficient tumors can be effectively minimized by using optimized PARP inhibitors.
Significance: In this study, we show that loss of 53BP1 causes resistance to PARP inhibition in mouse mammary tumors that are deficient in BRCA1. We hypothesize that low expression or absence of 53BP1 also reduces the response of patients with BRCA1-deficient tumors to PARP inhibitors.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
A. Cranston, N.M.B. Martin, A. Lau, and M.J. O’Connor were employees of KuDOS Pharmaceuticals, which developed olaparib and AZD2461. A. Lau and M.J. O’Connor are currently employees of AstraZeneca.
Figures



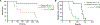
Comment in
-
Mechanisms of resistance to PARP inhibitors--three and counting.Cancer Discov. 2013 Jan;3(1):20-3. doi: 10.1158/2159-8290.CD-12-0514. Cancer Discov. 2013. PMID: 23319766 Free PMC article.
References
-
- Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005;434:913–7. - PubMed
-
- Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005;434:917–21. - PubMed
-
- McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res 2006;66:8109–15. - PubMed
-
- Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 2010;376:245–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

