EYA1-SIX1 complex in neurosensory cell fate induction in the mammalian inner ear
- PMID: 23104013
- PMCID: PMC6592049
- DOI: 10.1016/j.heares.2012.09.009
EYA1-SIX1 complex in neurosensory cell fate induction in the mammalian inner ear
Abstract
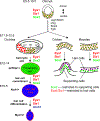
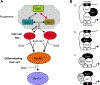
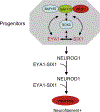
The phosphatase-transactivator EYA1 interacts with the homeodomain protein SIX1 to form transcriptional activation complexes, which play essential roles in regulating cell proliferation, survival and induction of sensory and neuronal differentiation programs during inner ear development. Mutations of the Eya1 and Six1 genes cause profound developmental auditory defects in mice and humans. The molecular mechanisms and developmental processes controlled by the EYA1 and SIX1 complex in inner ear development and neurosensory fate induction are the focus of this review.
Published by Elsevier B.V.
Figures

Similar articles
-
Eya1 is required for lineage-specific differentiation, but not for cell survival in the zebrafish adenohypophysis.Dev Biol. 2006 Apr 1;292(1):189-204. doi: 10.1016/j.ydbio.2005.12.036. Epub 2006 Feb 3. Dev Biol. 2006. PMID: 16458879
-
Eya1 regulates the growth of otic epithelium and interacts with Pax2 during the development of all sensory areas in the inner ear.Dev Biol. 2006 Oct 15;298(2):430-41. doi: 10.1016/j.ydbio.2006.06.049. Epub 2006 Jul 7. Dev Biol. 2006. PMID: 16916509 Free PMC article.
-
Eya1-Six1 interaction is sufficient to induce hair cell fate in the cochlea by activating Atoh1 expression in cooperation with Sox2.Dev Cell. 2012 Feb 14;22(2):377-90. doi: 10.1016/j.devcel.2011.12.006. Dev Cell. 2012. PMID: 22340499 Free PMC article.
-
The EYA-SO/SIX complex in development and disease.Pediatr Nephrol. 2013 Jun;28(6):843-54. doi: 10.1007/s00467-012-2246-1. Epub 2012 Jul 19. Pediatr Nephrol. 2013. PMID: 22806561 Free PMC article. Review.
-
Transcription factors that control inner ear development and their potential for transdifferentiation and reprogramming.Hear Res. 2013 Mar;297:84-90. doi: 10.1016/j.heares.2012.11.001. Epub 2012 Nov 14. Hear Res. 2013. PMID: 23159917 Review.
Cited by
-
Bioelectric signalling via potassium channels: a mechanism for craniofacial dysmorphogenesis in KCNJ2-associated Andersen-Tawil Syndrome.J Physiol. 2016 Jun 15;594(12):3245-70. doi: 10.1113/JP271930. Epub 2016 Apr 13. J Physiol. 2016. PMID: 26864374 Free PMC article.
-
Open chromatin dynamics in prosensory cells of the embryonic mouse cochlea.Sci Rep. 2019 Jun 21;9(1):9060. doi: 10.1038/s41598-019-45515-2. Sci Rep. 2019. PMID: 31227770 Free PMC article.
-
The transcriptional coactivator Eya1 exerts transcriptional repressive activity by interacting with REST corepressors and REST-binding sequences to maintain nephron progenitor identity.Nucleic Acids Res. 2022 Oct 14;50(18):10343-10359. doi: 10.1093/nar/gkac760. Nucleic Acids Res. 2022. PMID: 36130284 Free PMC article.
-
EYA1's Conformation Specificity in Dephosphorylating Phosphothreonine in Myc and Its Activity on Myc Stabilization in Breast Cancer.Mol Cell Biol. 2016 Dec 19;37(1):e00499-16. doi: 10.1128/MCB.00499-16. Print 2017 Jan 1. Mol Cell Biol. 2016. PMID: 27795300 Free PMC article.
-
Multiple Functions of the Eya Phosphotyrosine Phosphatase.Mol Cell Biol. 2015 Dec 14;36(5):668-77. doi: 10.1128/MCB.00976-15. Mol Cell Biol. 2015. PMID: 26667035 Free PMC article. Review.
References
-
- Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, Vincent C, Levi-Acobas F, Cruaud C, Le Merrer M, Mathieu M, Konig R, Vigneron J, Weissenbach J, Petit C, Weil D, 1997a. Clustering of mutations responsible for branchio-oto-renal (BOR) syndrome in the eyes absent homologous region (eyaHR) of EYA1. Hum. Mol. Genet 6, 2247–2255. - PubMed
-
- Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, Vincent C, Weil D, Cruaud C, Sahly I, Leibovici M, Bitner-Glindzicz M, Francis M, Lacombe D, Vigneron J, Charachon R, Boven K, Bedbeder P, Van Regemorter N, Weissenbach J, Petit C, 1997b. A human homologue of the Drosophila eyes absent gene underlies branchio-oto-renal (BOR) syndrome and identifies a novel gene family. Nat. Genet 15, 157–164. - PubMed
-
- Barald KF, Kelley MW, 2004. From placode to polarization: new tunes in inner ear development. Development 131, 4119–4130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

