Phylogenetic, metabolic, and taxonomic diversities shape mediterranean fruit fly microbiotas during ontogeny
- PMID: 23104413
- PMCID: PMC3536086
- DOI: 10.1128/AEM.02761-12
Phylogenetic, metabolic, and taxonomic diversities shape mediterranean fruit fly microbiotas during ontogeny
Abstract
The Mediterranean fruit fly (medfly) (Ceratitis capitata) lays eggs in fruits, where larvae subsequently develop, causing large-scale agricultural damage. Within its digestive tract, the fly supports an extended bacterial community that is composed of multiple strains of a variety of enterobacterial species. Most of these bacteria appear to be functionally redundant, with most strains sustaining diazotrophy and/or pectinolysis. At least some of these bacteria were shown to be vertically inherited, but colonization, structural, and metabolic aspects of the community's dynamics have not been investigated. We used fluorescent in situ hybridization, metabolic profiling, plate cultures, and pyrosequencing to show that an initial, egg-borne, diverse community expands throughout the fly's life cycle. While keeping "core" diazotrophic and pectinolytic functions, it also harbors diverse and fluctuating populations that express varied metabolic capabilities. We suggest that the metabolic and compositional plasticity of the fly's microbiota provides potential adaptive advantages to the medfly host and that its acquisition and dynamics are affected by mixed processes that include stochastic effects, host behavior, and molecular barriers.
Figures
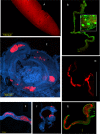

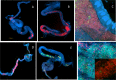
References
-
- Baumann P, Moran NA, Baumann L. 2006. Bacteriocyte-associated endosymbionts of insects, p 403–438 In Dworkin M, Rosenberg E, Schleifer K-H, Stackebrandt E. (ed), The prokaryotes. Springer, New York, NY
-
- Behar A, Jurkevitch E, Yuval B. 2008. Bringing back the fruit into fruit fly-bacteria interactions. Mol. Ecol. 17:1375–1386 - PubMed
-
- Ben Yosef M, Jurkevitch E, Yuval B. 2008. Effect of bacteria on nutritional status and reproductive success of the Mediterranean fruit fly Ceratitis capitata. Physiol. Entomol. 33:145–154
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

