Mitochondrial morphology, topology, and membrane interactions in skeletal muscle: a quantitative three-dimensional electron microscopy study
- PMID: 23104694
- PMCID: PMC3544498
- DOI: 10.1152/japplphysiol.01096.2012
Mitochondrial morphology, topology, and membrane interactions in skeletal muscle: a quantitative three-dimensional electron microscopy study
Abstract
Dynamic remodeling of mitochondrial morphology through membrane dynamics are linked to changes in mitochondrial and cellular function. Although mitochondrial membrane fusion/fission events are frequent in cell culture models, whether mitochondrial membranes dynamically interact in postmitotic muscle fibers in vivo remains unclear. Furthermore, a quantitative assessment of mitochondrial morphology in intact muscle is lacking. Here, using electron microscopy (EM), we provide evidence of interacting membranes from adjacent mitochondria in intact mouse skeletal muscle. Electron-dense mitochondrial contact sites consistent with events of outer mitochondrial membrane tethering are also described. These data suggest that mitochondrial membranes interact in vivo among mitochondria, possibly to induce morphology transitions, for kiss-and-run behavior, or other processes involving contact between mitochondrial membranes. Furthermore, a combination of freeze-fracture scanning EM and transmission EM in orthogonal planes was used to characterize and quantify mitochondrial morphology. Two subpopulations of mitochondria were studied: subsarcolemmal (SS) and intermyofibrillar (IMF), which exhibited significant differences in morphological descriptors, including form factor (means ± SD for SS: 1.41 ± 0.45 vs. IMF: 2.89 ± 1.76, P < 0.01) and aspect ratio (1.97 ± 0.83 vs. 3.63 ± 2.13, P < 0.01) and circularity (0.75 ± 0.16 vs. 0.45 ± 0.22, P < 0.01) but not size (0.28 ± 0.31 vs. 0.27 ± 0.20 μm(2)). Frequency distributions for mitochondrial size and morphological parameters were highly skewed, suggesting the presence of mechanisms to influence mitochondrial size and shape. In addition, physical continuities between SS and IMF mitochondria indicated mixing of both subpopulations. These data provide evidence that mitochondrial membranes interact in vivo in mouse skeletal muscle and that factors may be involved in regulating skeletal muscle mitochondrial morphology.
Figures

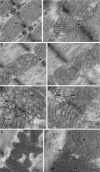
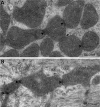



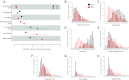
Comment in
-
Muscle mitochondrial ultrastructure: new insights into morphological divergences.J Appl Physiol (1985). 2013 Jan 15;114(2):159-60. doi: 10.1152/japplphysiol.01428.2012. Epub 2012 Nov 29. J Appl Physiol (1985). 2013. PMID: 23195634 No abstract available.
References
-
- Adhihetty PJ, Ljubicic V, Menzies KJ, Hood DA. Differential susceptibility of subsarcolemmal and intermyofibrillar mitochondria to apoptotic stimuli. Am J Physiol Cell Physiol 289: C994–C1001, 2005 - PubMed
-
- Bach D, Pich S, Soriano FX, Vega N, Baumgartner B, Oriola J, Daugaard JR, Lloberas J, Camps M, Zierath JR, Rabasa-Lhoret R, Wallberg-Henriksson H, Laville M, Palacin M, Vidal H, Rivera F, Brand M, Zorzano A. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem 278: 17190–17197, 2003 - PubMed
-
- Bakeeva LE, Chentsov Yu S, Skulachev VP. Intermitochondrial contacts in myocardiocytes. J Mol Cell Cardiol 15: 413–420, 1983 - PubMed
-
- Bakeeva LE, Chentsov Yu S, Skulachev VP. Mitochondrial framework (reticulum mitochondriale) in rat diaphragm muscle. Biochim Biophys Acta 501: 349–369, 1978 - PubMed
-
- Bubenzer HJ. [The thin and the thick muscular fibers of the rat diaphragm]. Z Zellforsch Mikrosk Anat 69: 520–550, 1966 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

