Involvement of GDH3-encoded NADP+-dependent glutamate dehydrogenase in yeast cell resistance to stress-induced apoptosis in stationary phase cells
- PMID: 23105103
- PMCID: PMC3531738
- DOI: 10.1074/jbc.M112.375360
Involvement of GDH3-encoded NADP+-dependent glutamate dehydrogenase in yeast cell resistance to stress-induced apoptosis in stationary phase cells
Abstract
Glutamate metabolism is linked to a number of fundamental metabolic pathways such as amino acid metabolism, the TCA cycle, and glutathione (GSH) synthesis. In the yeast Saccharomyces cerevisiae, glutamate is synthesized from α-ketoglutarate by two NADP(+)-dependent glutamate dehydrogenases (NADP-GDH) encoded by GDH1 and GDH3. Here, we report the relationship between the function of the NADP-GDH and stress-induced apoptosis. Gdh3-null cells showed accelerated chronological aging and hypersusceptibility to thermal and oxidative stress during stationary phase. Upon exposure to oxidative stress, Gdh3-null strains displayed a rapid loss in viability associated with typical apoptotic hallmarks, i.e. reactive oxygen species accumulation, nuclear fragmentation, DNA breakage, and phosphatidylserine translocation. In addition, Gdh3-null cells, but not Gdh1-null cells, had a higher tendency toward GSH depletion and subsequent reactive oxygen species accumulation than did WT cells. GSH depletion was rescued by exogenous GSH or glutamate. The hypersusceptibility of stationary phase Gdh3-null cells to stress-induced apoptosis was suppressed by deletion of GDH2. Promoter swapping and site-directed mutagenesis of GDH1 and GDH3 indicated that the necessity of GDH3 for the resistance to stress-induced apoptosis and chronological aging is due to the stationary phase-specific expression of GDH3 and concurrent degradation of Gdh1 in which the Lys-426 residue plays an essential role.
Figures




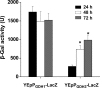

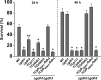
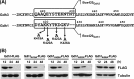
Similar articles
-
The NADP+-dependent glutamate dehydrogenase Gdh1 is subjected to glucose starvation-induced reversible aggregation that affects stress resistance in yeast.J Microbiol. 2019 Oct;57(10):884-892. doi: 10.1007/s12275-019-9065-z. Epub 2019 Aug 3. J Microbiol. 2019. PMID: 31376105
-
Swi/SNF-GCN5-dependent chromatin remodelling determines induced expression of GDH3, one of the paralogous genes responsible for ammonium assimilation and glutamate biosynthesis in Saccharomyces cerevisiae.Mol Microbiol. 2005 Jul;57(1):291-305. doi: 10.1111/j.1365-2958.2005.04689.x. Mol Microbiol. 2005. PMID: 15948967
-
NADP-glutamate dehydrogenase isoenzymes of Saccharomyces cerevisiae. Purification, kinetic properties, and physiological roles.J Biol Chem. 2001 Nov 23;276(47):43775-83. doi: 10.1074/jbc.M107986200. Epub 2001 Sep 18. J Biol Chem. 2001. PMID: 11562373
-
The pleiotropic effects of the glutamate dehydrogenase (GDH) pathway in Saccharomyces cerevisiae.Microb Cell Fact. 2018 Nov 1;17(1):170. doi: 10.1186/s12934-018-1018-4. Microb Cell Fact. 2018. PMID: 30384856 Free PMC article. Review.
-
Lifting the veil on tumor metabolism: A GDH1-focused perspective.iScience. 2025 May 3;28(6):112551. doi: 10.1016/j.isci.2025.112551. eCollection 2025 Jun 20. iScience. 2025. PMID: 40487432 Free PMC article. Review.
Cited by
-
Redox engineering by ectopic expression of glutamate dehydrogenase genes links NADPH availability and NADH oxidation with cold growth in Saccharomyces cerevisiae.Microb Cell Fact. 2015 Jul 9;14:100. doi: 10.1186/s12934-015-0289-2. Microb Cell Fact. 2015. PMID: 26156706 Free PMC article.
-
Proteolytic clipping of histone tails: the emerging role of histone proteases in regulation of various biological processes.Mol Biol Rep. 2014 May;41(5):2717-30. doi: 10.1007/s11033-014-3181-y. Mol Biol Rep. 2014. PMID: 24469733 Review.
-
Diversification of the kinetic properties of yeast NADP-glutamate-dehydrogenase isozymes proceeds independently of their evolutionary origin.Microbiologyopen. 2017 Apr;6(2):e00419. doi: 10.1002/mbo3.419. Epub 2016 Nov 19. Microbiologyopen. 2017. PMID: 27864882 Free PMC article.
-
Genome-Wide Transcription Study of Cryptococcus neoformans H99 Clinical Strain versus Environmental Strains.PLoS One. 2015 Sep 11;10(9):e0137457. doi: 10.1371/journal.pone.0137457. eCollection 2015. PLoS One. 2015. PMID: 26360021 Free PMC article.
-
The SrkA Kinase Is Part of the SakA Mitogen-Activated Protein Kinase Interactome and Regulates Stress Responses and Development in Aspergillus nidulans.Eukaryot Cell. 2015 May;14(5):495-510. doi: 10.1128/EC.00277-14. Epub 2015 Mar 27. Eukaryot Cell. 2015. PMID: 25820520 Free PMC article.
References
-
- Hudson R. C., Daniel R. M. (1993) l-Glutamate dehydrogenases: distribution, properties, and mechanism. Comp. Biochem. Physiol. B 106, 767–792 - PubMed
-
- McKenna M. C., Sonnewald U., Huang X., Stevenson J., Zielke H. R. (1996) Exogenous glutamate concentration regulates the metabolic fate of glutamate in astrocytes. J. Neurochem. 66, 386–393 - PubMed
-
- Plaitakis A., Zaganas I. (2001) Regulation of human glutamate dehydrogenases: implications for glutamate, ammonia, and energy metabolism in brain. J. Neurosci. Res. 66, 899–908 - PubMed
-
- Cooper A. J., Plum F. (1987) Biochemistry and physiology of brain ammonia. Physiol. Rev. 67, 440–519 - PubMed
-
- Plaitakis A., Metaxari M., Shashidharan P. (2000) Nerve tissue-specific (GLUD2) and housekeeping (GLUD1) human glutamate dehydrogenases are regulated by distinct allosteric mechanisms: implications for biologic function. J. Neurochem. 75, 1862–1869 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

