Involvement of GDH3-encoded NADP+-dependent glutamate dehydrogenase in yeast cell resistance to stress-induced apoptosis in stationary phase cells
- PMID: 23105103
- PMCID: PMC3531738
- DOI: 10.1074/jbc.M112.375360
Involvement of GDH3-encoded NADP+-dependent glutamate dehydrogenase in yeast cell resistance to stress-induced apoptosis in stationary phase cells
Abstract
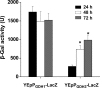
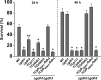
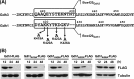
Glutamate metabolism is linked to a number of fundamental metabolic pathways such as amino acid metabolism, the TCA cycle, and glutathione (GSH) synthesis. In the yeast Saccharomyces cerevisiae, glutamate is synthesized from α-ketoglutarate by two NADP(+)-dependent glutamate dehydrogenases (NADP-GDH) encoded by GDH1 and GDH3. Here, we report the relationship between the function of the NADP-GDH and stress-induced apoptosis. Gdh3-null cells showed accelerated chronological aging and hypersusceptibility to thermal and oxidative stress during stationary phase. Upon exposure to oxidative stress, Gdh3-null strains displayed a rapid loss in viability associated with typical apoptotic hallmarks, i.e. reactive oxygen species accumulation, nuclear fragmentation, DNA breakage, and phosphatidylserine translocation. In addition, Gdh3-null cells, but not Gdh1-null cells, had a higher tendency toward GSH depletion and subsequent reactive oxygen species accumulation than did WT cells. GSH depletion was rescued by exogenous GSH or glutamate. The hypersusceptibility of stationary phase Gdh3-null cells to stress-induced apoptosis was suppressed by deletion of GDH2. Promoter swapping and site-directed mutagenesis of GDH1 and GDH3 indicated that the necessity of GDH3 for the resistance to stress-induced apoptosis and chronological aging is due to the stationary phase-specific expression of GDH3 and concurrent degradation of Gdh1 in which the Lys-426 residue plays an essential role.
Figures





References
-
- Hudson R. C., Daniel R. M. (1993) l-Glutamate dehydrogenases: distribution, properties, and mechanism. Comp. Biochem. Physiol. B 106, 767–792 - PubMed
-
- McKenna M. C., Sonnewald U., Huang X., Stevenson J., Zielke H. R. (1996) Exogenous glutamate concentration regulates the metabolic fate of glutamate in astrocytes. J. Neurochem. 66, 386–393 - PubMed
-
- Plaitakis A., Zaganas I. (2001) Regulation of human glutamate dehydrogenases: implications for glutamate, ammonia, and energy metabolism in brain. J. Neurosci. Res. 66, 899–908 - PubMed
-
- Cooper A. J., Plum F. (1987) Biochemistry and physiology of brain ammonia. Physiol. Rev. 67, 440–519 - PubMed
-
- Plaitakis A., Metaxari M., Shashidharan P. (2000) Nerve tissue-specific (GLUD2) and housekeeping (GLUD1) human glutamate dehydrogenases are regulated by distinct allosteric mechanisms: implications for biologic function. J. Neurochem. 75, 1862–1869 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

