Pegylated Interferon alfa-2a monotherapy results in suppression of HIV type 1 replication and decreased cell-associated HIV DNA integration
- PMID: 23105144
- PMCID: PMC3532820
- DOI: 10.1093/infdis/jis663
Pegylated Interferon alfa-2a monotherapy results in suppression of HIV type 1 replication and decreased cell-associated HIV DNA integration
Abstract
Background: Antiretroviral therapy (ART)-mediated immune reconstitution fails to restore the capacity of the immune system to spontaneously control human immunodeficiency virus (HIV) replication.
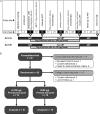
Methods: A total of 23 HIV type 1 (HIV-1)-infected, virologically suppressed subjects receiving ART (CD4(+) T-cell count, >450 cells/μL) were randomly assigned to have 180 μg/week (for arm A) or 90 μg/week (for arm B) of pegylated (Peg) interferon alfa-2a added to their current ART regimen. After 5 weeks, ART was interrupted, and Peg-interferon alfa-2a was continued for up to 12 weeks (the primary end point), with an option to continue to 24 weeks. End points included virologic failure (viral load, ≥ 400 copies/mL) and adverse events. Residual viral load and HIV-1 DNA integration were also assessed.
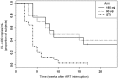
Results: At week 12 of Peg-interferon alfa-2a monotherapy, viral suppression was observed in 9 of 20 subjects (45%), a significantly greater proportion than expected (arm A, P = .0088; arm B, P = .0010; combined arms, P < .0001). Over 24 weeks, both arms had lower proportions of subjects who had viral load, compared with the proportion of subjects in a historical control group (arm A, P = .0046; arm B, P = .0011). Subjects who had a sustained viral load of <400 copies/mL had decreased levels of integrated HIV DNA (P = .0313) but increased residual viral loads (P = .0078), compared with subjects who experienced end-point failure.
Conclusions: Peg-interferon alfa-2a immunotherapy resulted in control of HIV replication and decreased HIV-1 integration, supporting a role for immunomediated approaches in HIV suppression and/or eradication.
Clinical trials registration: NCT00594880.
Figures


Comment in
-
Interferon alfa therapy: toward an improved treatment for HIV infection.J Infect Dis. 2013 Jan 15;207(2):201-3. doi: 10.1093/infdis/jis667. Epub 2012 Oct 26. J Infect Dis. 2013. PMID: 23105145 No abstract available.
-
Improved treatment for primary HIV infection by interferon-alfa therapy? Does HCV treatment in HIV/HCV coinfected patients help us to test this hypothesis? Reply to zur Wiesch and van Lunzen.J Infect Dis. 2013 Jul 15;208(2):363. doi: 10.1093/infdis/jit160. Epub 2013 Apr 9. J Infect Dis. 2013. PMID: 23570845 No abstract available.
-
Improved treatment for primary HIV infection by interferon-alfa therapy? Does HCV treatment in HIV/HCV coinfected patients help us to test this hypothesis?J Infect Dis. 2013 Jul 15;208(2):362-3. doi: 10.1093/infdis/jit159. Epub 2013 Apr 9. J Infect Dis. 2013. PMID: 23570847 No abstract available.
-
Interferon alfa partially inhibits HIV replication in hepatocytes in vitro.J Infect Dis. 2013 Sep 1;208(5):865-6. doi: 10.1093/infdis/jit255. Epub 2013 Jun 10. J Infect Dis. 2013. PMID: 23757338 No abstract available.
References
-
- Chun TW, Carruth L, Finzi D, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–8. - PubMed
-
- Angel JB, Routy JP, Tremblay C, et al. A randomized controlled trial of HIV therapeutic vaccination using ALVAC with or without Remune. AIDS. 2011;25:731–9. - PubMed
-
- Henry K, Katzenstein D, Cherng DW, et al. A pilot study evaluating time to CD4 T-cell count <350 cells/mm(3) after treatment interruption following antiretroviral therapy +/- interleukin 2: results of ACTG A5102. J Acquir Immune Defic Syndr. 2006;42:140–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- K08 AI073102/AI/NIAID NIH HHS/United States
- P30 CA010815/CA/NCI NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- K02 AI078766/AI/NIAID NIH HHS/United States
- K08AI073102/AI/NIAID NIH HHS/United States
- HHSN261200800001C/RC/CCR NIH HHS/United States
- K24 AI069994/AI/NIAID NIH HHS/United States
- U01AI065279/AI/NIAID NIH HHS/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- U01 AI065279/AI/NIAID NIH HHS/United States
- R21 AI087461/AI/NIAID NIH HHS/United States
- P30 CA10815/CA/NCI NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- P30 AI 045008/AI/NIAID NIH HHS/United States
- HHSN261200800001E/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

