H(2)azapa: a versatile acyclic multifunctional chelator for (67)Ga, (64)Cu, (111)In, and (177)Lu
- PMID: 23106422
- PMCID: PMC4968691
- DOI: 10.1021/ic302225z
H(2)azapa: a versatile acyclic multifunctional chelator for (67)Ga, (64)Cu, (111)In, and (177)Lu
Abstract
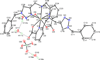
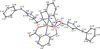
Preliminary experiments with the novel acyclic triazole-containing bifunctional chelator H2azapa and the radiometals (64)Cu, (67)Ga, (111)In, and (177)Lu have established its significant versatile potential as an alternative to 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) for metal-based radiopharmaceuticals. Unlike DOTA, H2azapa radiolabels quantitatively with (64)Cu, (67)Ga, (111)In, and (177)Lu in 10 min at room temperature. In vitro competition experiments with human blood serum show that (64)Cu remained predominantly chelate-bound, with only 2% transchelated to serum proteins after 20 h. Biodistribution experiments with [(64)Cu(azapa)] in mice reveal uptake in various organs, particularly in the liver, lungs, heart, intestines, and kidneys. When compared to [(64)Cu(DOTA)](2-), the lipophilic neutral [(64)Cu(azapa)] was cleared through the gastrointestinal tract and accumulated in the liver, which is common for lipophilic compounds or free (64)Cu. The chelator H2azapa is a model complex for a click-based bifunctional chelating agent, and the lipophilic benzyl "place-holders" will be replaced by hydrophilic peptides to modulate the pharmacokinetics and direct activity away from the liver and gut. The solid-state molecular structure of [In(azapa)(H2O)][ClO4] reveals a very rare eight-coordinate distorted square antiprismatic geometry with one triazole arm bound, and the structure of [(64)Cu(azapa)] shows a distorted octahedral geometry. The present study demonstrates significant potential for bioconjugates of H2azapa as alternatives to DOTA in copper-based radiopharmaceuticals, with the highly modular and "clickable" molecular scaffold of H2azapa easily modified into a variety of bioconjugates. H2azapa is a versatile addition to the "pa" family, joining the previously published H2dedpa ((67/68)Ga and (64)Cu), H4octapa ((111)In, (177)Lu, and (90)Y), and H5decapa ((225)Ac) to cover a wide range of important nuclides.
Figures


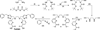

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

