Neonatal neurobehavior effects following buprenorphine versus methadone exposure
- PMID: 23106928
- PMCID: PMC4337995
- DOI: 10.1111/j.1360-0443.2012.04040.x
Neonatal neurobehavior effects following buprenorphine versus methadone exposure
Abstract
Aim: To determine the effects of in utero exposure to methadone or buprenorphine on infant neurobehavior.
Design: Three sites from the Maternal Opioid Treatment: Human Experimental Research (MOTHER) study, a double-blind, double-dummy, randomized clinical trial participated in this substudy.
Setting: Medical Centers that provided comprehensive maternal care to opioid-dependent pregnant women in Baltimore, MD, Providence, RI and Vienna, Austria.
Participants: Thirty-nine full-term infants.
Measurements: The Neonatal Intensive Care Unit (NICU) Network Neurobehavioral Scale (NNNS) was administered to a subgroup of infants on postpartum days 3, 5, 7, 10, 14-15 and 28-30.
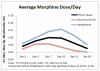
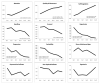
Findings: While neurobehavior improved for both medication conditions over time, infants exposed in utero to buprenorphine exhibited fewer stress-abstinence signs (P < 0.001), were less excitable (P < 0.001) and less over-aroused (P < 0.01), exhibited less hypertonia (P < 0.007), had better self-regulation (P < 0.04) and required less handling (P < 0.001) to maintain a quiet alert state relative to in utero methadone-exposed infants. Infants who were older when they began morphine treatment for withdrawal had higher self-regulation scores (P < 0.01), and demonstrated the least amount of excitability (P < 0.02) and hypertonia (P < 0.02) on average. Quality of movement was correlated negatively with peak NAS score (P < 0.01), number of days treated with morphine for NAS (P < 0.01) and total amount of morphine received (P < 0.03). Excitability scores were related positively to total morphine dose (P < 0.03).
Conclusion: While neurobehavior improves during the first month of postnatal life for in utero agonist medication-exposed neonates, buprenorphine exposure results in superior neurobehavioral scores and less severe withdrawal than does methadone exposure.
Trial registration: ClinicalTrials.gov NCT00271219.
© 2012 The Authors, Addiction © 2012 Society for the Study of Addiction.
Figures
References
-
- Johnson RE, Jones HE, Jasinski DR, Svikis DS, Haug NA, Jansson LM, et al. Buprenorphine treatment of pregnant opioid-dependent women: maternal and neonatal outcomes. Drug Alcoh Dep. 2001;63:97–103. - PubMed
-
- Jones HE, Johnson RE, Jasinski DR, O’Grady KE, Chisholm CA, Choo RE, et al. Buprenorphine versus methadone in the treatment of pregnant opioid-dependent patients: effects on the neonatal abstinence syndrome. Drug Alcohol Dep. 2005;79:1–10. - PubMed
-
- Fischer G, Johnson RE, Eder H, Jagsch R, Peternell A, Weninger M, et al. Treatment of opioid-dependent pregnant women with Buprenorphine. Addiction. 2000;95:239–244. - PubMed
-
- Lejeune C, Simmat-Durand L, Gourarier L, Aubisson S, Groupe d’Etudes Grossesse et Addictions (GEGA) Prospective multicenter observational study of 260 infants born to 259 opiate-dependent mothers on methadone or high-dose buprenorphine substitution. Drug Alcohol Depend. 2006;82:250–57. - PubMed
-
- Fischer G, Ortner R, Rohrmeister K, Jagsch R, Baewert A, Langer M, et al. Methadone versus buprenorphine in pregnant addicts: A double-blind, double-dummy comparison study. Addiction. 2006;101:275–81. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- R01 DA 015738/DA/NIDA NIH HHS/United States
- R01 DA 018417/DA/NIDA NIH HHS/United States
- R01 DA 15832/DA/NIDA NIH HHS/United States
- R01 DA015764/DA/NIDA NIH HHS/United States
- R01 DA 015778/DA/NIDA NIH HHS/United States
- M01 RR000109/RR/NCRR NIH HHS/United States
- M01 RR 00095/RR/NCRR NIH HHS/United States
- R01 DA018417/DA/NIDA NIH HHS/United States
- R01 DA 015741/DA/NIDA NIH HHS/United States
- R01 DA 015764/DA/NIDA NIH HHS/United States
- R01 DA015778/DA/NIDA NIH HHS/United States
- R01 DA 018410/DA/NIDA NIH HHS/United States
- M01 RR 109/RR/NCRR NIH HHS/United States
- R01 DA 017513/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical