Muscle-fiber transdifferentiation in an experimental model of respiratory chain myopathy
- PMID: 23107834
- PMCID: PMC3580545
- DOI: 10.1186/ar4076
Muscle-fiber transdifferentiation in an experimental model of respiratory chain myopathy
Abstract
Introduction: Skeletal muscle fiber composition and muscle energetics are not static and change in muscle disease. This study was performed to determine whether a mitochondrial myopathy is associated with adjustments in skeletal muscle fiber-type composition.
Methods: Ten rats were treated with zidovudine, an antiretroviral nucleoside reverse transcriptase inhibitor that induces a myopathy by interfering with mitochondrial functions. Soleus muscles were examined after 21 weeks of treatment. Ten untreated rats served as controls.
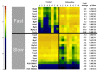
Results: Zidovudine induced a myopathy with mitochondrial DNA depletion, abnormalities in mitochondrial ultrastructure, and reduced cytochrome c oxidase activity. Mitochondrial DNA was disproportionally more diminished in type I compared with type II fibers, whereas atrophy predominated in type II fibers. Compared with those of controls, zidovudine-exposed soleus muscles contained an increased proportion (256%) of type II fibers, whereas neonatal myosin heavy chains remained repressed, indicating fiber-type transformation in the absence of regeneration. Microarray gene-expression analysis confirmed enhanced fast-fiber isoforms, repressed slow-fiber transcripts, and reduced neonatal fiber transcripts in the mitochondrial myopathy. Respiratory chain transcripts were diminished, whereas the enzymes of glycolysis and glycogenolysis were enhanced, indicating a metabolic adjustment from oxidative to glycolytic capacities. A coordinated regulation was found of transcription factors known to orchestrate type II fiber formation (upregulation of MyoD, Six1, Six2, Eya1, and Sox6, and downregulation of myogenin and ERRγ).
Conclusions: The type I to type II fiber transformation in mitochondrial myopathy implicates mitochondrial function as a new regulator of skeletal muscle fiber type.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

