The diacylglycerol lipases: structure, regulation and roles in and beyond endocannabinoid signalling
- PMID: 23108545
- PMCID: PMC3481529
- DOI: 10.1098/rstb.2011.0387
The diacylglycerol lipases: structure, regulation and roles in and beyond endocannabinoid signalling
Abstract
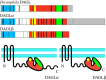
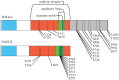
The diacylglycerol lipases (DAGLs) hydrolyse diacylglycerol to generate 2-arachidonoylglycerol (2-AG), the most abundant ligand for the CB(1) and CB(2) cannabinoid receptors in the body. DAGL-dependent endocannabinoid signalling regulates axonal growth and guidance during development, and is required for the generation and migration of new neurons in the adult brain. At developed synapses, 2-AG released from postsynaptic terminals acts back on presynaptic CB(1) receptors to inhibit the secretion of both excitatory and inhibitory neurotransmitters, with this DAGL-dependent synaptic plasticity operating throughout the nervous system. Importantly, the DAGLs have functions that do not involve cannabinoid receptors. For example, 2-AG is the precursor of arachidonic acid in a pathway that maintains the level of this essential lipid in the brain and other organs. This pathway also drives the cyclooxygenase-dependent generation of inflammatory prostaglandins in the brain, which has recently been implicated in the degeneration of dopaminergic neurons in Parkinson's disease. Remarkably, we still know very little about the mechanisms that regulate DAGL activity-however, key insights can be gleaned by homology modelling against other α/β hydrolases and from a detailed examination of published proteomic studies and other databases. These identify a regulatory loop with a highly conserved signature motif, as well as phosphorylation and palmitoylation as post-translational mechanisms likely to regulate function.
Figures

References
-
- Brose N., Betz A., Wegmeyer H. 2004. Divergent and convergent signaling by the diacylglycerol second messenger pathway in mammals. Curr. Opin. Neurobiol. 14, 328–340 10.1016/j.conb.2004.05.006 (doi:10.1016/j.conb.2004.05.006) - DOI - PubMed
-
- Carrasco S., Merida I. 2007. Diacylglycerol, when simplicity becomes complex. Trends Biochem. Sci. 32, 27–36 10.1016/j.tibs.2006.11.004 (doi:10.1016/j.tibs.2006.11.004) - DOI - PubMed
-
- Prescott S. M., Majerus P. W. 1983. Characterization of 1,2-diacylglycerol hydrolysis in human platelets. Demonstration of an arachidonoyl-monoacylglycerol intermediate. J. Biol. Chem. 258, 764–769 - PubMed
-
- Allen A. C., Gammon C. M., Ousley A. H., McCarthy K. D., Morell P. 1992. Bradykinin stimulates arachidonic acid release through the sequential actions of an sn-1 diacylglycerol lipase and a monoacylglycerol lipase. J. Neurochem. 58, 1130–1139 10.1111/j.1471-4159.1992.tb09372.x (doi:10.1111/j.1471-4159.1992.tb09372.x) - DOI - PubMed
-
- Kennerly D. A., Sullivan T. J., Sylwester P., Parker C. W. 1979. Diacylglycerol metabolism in mast cells: a potential role in membrane fusion and arachidonic acid release. J. Exp. Med. 150, 1039–1044 10.1084/jem.150.4.1039 (doi:10.1084/jem.150.4.1039) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
