Effects of in vivo injury on the neuromuscular junction in healthy and dystrophic muscles
- PMID: 23109110
- PMCID: PMC3577526
- DOI: 10.1113/jphysiol.2012.241679
Effects of in vivo injury on the neuromuscular junction in healthy and dystrophic muscles
Abstract
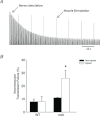
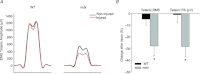
The most common and severe form of muscular dystrophy is Duchenne muscular dystrophy (DMD), a disorder caused by the absence of dystrophin, a structural protein found on the cytoplasmic surface of the sarcolemma of striated muscle fibres. Considerable attention has been dedicated to studying myofibre damage and muscle plasticity, but there is little information to determine if damage from contraction-induced injury occurs at or near the nerve terminal axon. We used α-bungarotoxin to compare neuromuscular junction (NMJ) morphology in healthy (wild-type, WT) and dystrophic (mdx) mouse quadriceps muscles and evaluated transcript levels of the post-synaptic muscle-specific kinase signalling complex. Our focus was to study changes in NMJs after injury induced with an established in vivo animal injury model. Neuromuscular transmission, electromyography (EMG), and NMJ morphology were assessed 24 h after injury. In non-injured muscle, muscle-specific kinase expression was significantly decreased in mdx compared to WT. Injury resulted in a significant loss of maximal torque in WT (39 ± 6%) and mdx (76 ± 8%) quadriceps, but significant changes in NMJ morphology, neuromuscular transmission and EMG data were found only in mdx following injury. Compared with WT mice, motor end-plates of mdx mice demonstrated less continuous morphology, more disperse acetylcholine receptor aggregates and increased number of individual acetylcholine receptor clusters, an effect that was exacerbated following injury. Neuromuscular transmission failure increased and the EMG measures decreased after injury in mdx mice only. The data show that eccentric contraction-induced injury causes morphological and functional changes to the NMJs in mdx skeletal muscle, which may play a role in excitation-contraction coupling failure and progression of the dystrophic process.
Figures



Similar articles
-
Recovery of altered neuromuscular junction morphology and muscle function in mdx mice after injury.Cell Mol Life Sci. 2015 Jan;72(1):153-64. doi: 10.1007/s00018-014-1663-7. Epub 2014 Jun 20. Cell Mol Life Sci. 2015. PMID: 24947322 Free PMC article.
-
Characterization of neuromuscular synapse function abnormalities in multiple Duchenne muscular dystrophy mouse models.Eur J Neurosci. 2016 Jun;43(12):1623-35. doi: 10.1111/ejn.13249. Epub 2016 May 9. Eur J Neurosci. 2016. PMID: 27037492
-
Imaging Analysis of the Neuromuscular Junction in Dystrophic Muscle.Methods Mol Biol. 2018;1687:57-72. doi: 10.1007/978-1-4939-7374-3_5. Methods Mol Biol. 2018. PMID: 29067656
-
Alterations of neuromuscular junctions in Duchenne muscular dystrophy.Neurosci Lett. 2020 Oct 15;737:135304. doi: 10.1016/j.neulet.2020.135304. Epub 2020 Aug 17. Neurosci Lett. 2020. PMID: 32818587 Free PMC article. Review.
-
Recent insights into neuromuscular junction biology in Duchenne muscular dystrophy: Impacts, challenges, and opportunities.EBioMedicine. 2020 Nov;61:103032. doi: 10.1016/j.ebiom.2020.103032. Epub 2020 Oct 8. EBioMedicine. 2020. PMID: 33039707 Free PMC article. Review.
Cited by
-
Acute failure of action potential conduction in mdx muscle reveals new mechanism of contraction-induced force loss.J Physiol. 2013 Aug 1;591(15):3765-76. doi: 10.1113/jphysiol.2013.254656. Epub 2013 Jun 10. J Physiol. 2013. PMID: 23753524 Free PMC article.
-
Eccentric exercise in aging and diseased skeletal muscle: good or bad?J Appl Physiol (1985). 2014 Jun 1;116(11):1439-45. doi: 10.1152/japplphysiol.00174.2013. Epub 2013 Mar 7. J Appl Physiol (1985). 2014. PMID: 23471953 Free PMC article. Review.
-
Effects of Zusanli and Ashi Acupoint Electroacupuncture on Repair of Skeletal Muscle and Neuromuscular Junction in a Rabbit Gastrocnemius Contusion Model.Evid Based Complement Alternat Med. 2016;2016:7074563. doi: 10.1155/2016/7074563. Epub 2016 Apr 13. Evid Based Complement Alternat Med. 2016. PMID: 27190536 Free PMC article.
-
Critical Role of Intracellular RyR1 Calcium Release Channels in Skeletal Muscle Function and Disease.Front Physiol. 2016 Jan 12;6:420. doi: 10.3389/fphys.2015.00420. eCollection 2015. Front Physiol. 2016. PMID: 26793121 Free PMC article. Review.
-
Electrical Impedance Myography to Detect the Effects of Electrical Muscle Stimulation in Wild Type and Mdx Mice.PLoS One. 2016 Mar 17;11(3):e0151415. doi: 10.1371/journal.pone.0151415. eCollection 2016. PLoS One. 2016. PMID: 26986564 Free PMC article.
References
-
- Aldrich TK, Shander A, Chaudhry I, Nagashima H. Fatigue of isolated rat diaphragm: role of impaired neuromuscular transmission. J Appl Physiol. 1986;61:1077–1083. - PubMed
-
- Batchelor CL, Winder SJ. Sparks, signals and shock absorbers: how dystrophin loss causes muscular dystrophy. Trends Cell Biol. 2006;16:198–205. - PubMed
-
- Bloch RJ, Gonzalez-Serratos H. Lateral force transmission across costameres in skeletal muscle. Exerc Sport Sci Rev. 2003;31:73–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

