Neuron-to-neuron transmission of α-synuclein fibrils through axonal transport
- PMID: 23109146
- PMCID: PMC3490229
- DOI: 10.1002/ana.23747
Neuron-to-neuron transmission of α-synuclein fibrils through axonal transport
Abstract
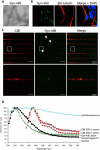
Objective: The lesions of Parkinson disease spread through the brain in a characteristic pattern that corresponds to axonal projections. Previous observations suggest that misfolded α-synuclein could behave as a prion, moving from neuron to neuron and causing endogenous α-synuclein to misfold. Here, we characterized and quantified the axonal transport of α-synuclein fibrils and showed that fibrils could be transferred from axons to second-order neurons following anterograde transport.
Methods: We grew primary cortical mouse neurons in microfluidic devices to separate somata from axonal projections in fluidically isolated microenvironments. We used live-cell imaging and immunofluorescence to characterize the transport of fluorescent α-synuclein fibrils and their transfer to second-order neurons.
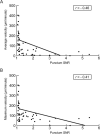
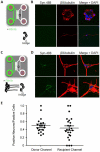
Results: Fibrillar α-synuclein was internalized by primary neurons and transported in axons with kinetics consistent with slow component-b of axonal transport (fast axonal transport with saltatory movement). Fibrillar α-synuclein was readily observed in the cell bodies of second-order neurons following anterograde axonal transport. Axon-to-soma transfer appeared not to require synaptic contacts.
Interpretation: These results support the hypothesis that the progression of Parkinson disease can be caused by neuron-to-neuron spread of α-synuclein aggregates and that the anatomical pattern of progression of lesions between axonally connected areas results from the axonal transport of such aggregates. That the transfer did not appear to be trans-synaptic gives hope that α-synuclein fibrils could be intercepted by drugs during the extracellular phase of their journey.
Copyright © 2012 American Neurological Association.
Figures

References
-
- Lees AJ. The Parkinson chimera. Neurology. 2009;72:S2–S11.2. - PubMed
-
- Brundin P, Li JY, Holton JL, Lindvall O, Revesz T. Research in motion: the enigma of Parkinson's disease pathology spread. Nat Reviews Neurosci. 2008;9:741–745. - PubMed
-
- Goedert M, Clavaguera F, Tolnay M. The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci. 2010;33:317–325. - PubMed
-
- Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–1320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

