Acute toxicities of unrelated bone marrow versus peripheral blood stem cell donation: results of a prospective trial from the National Marrow Donor Program
- PMID: 23109243
- PMCID: PMC3538330
- DOI: 10.1182/blood-2012-03-417667
Acute toxicities of unrelated bone marrow versus peripheral blood stem cell donation: results of a prospective trial from the National Marrow Donor Program
Erratum in
- Blood. 2014 Mar 20;123(12):1970
Abstract
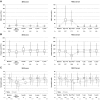
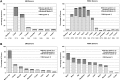
Although peripheral blood stem cells (PBSCs) have replaced bone marrow (BM) as the most common unrelated donor progenitor cell product collected, a direct comparison of concurrent PBSC versus BM donation experiences has not been performed. We report a prospective study of 2726 BM and 6768 PBSC donors who underwent collection from 2004 to 2009. Pain and toxicities were assessed at baseline, during G-CSF administration, on the day of collection, within 48 hours of donation, and weekly until full recovery. Peak levels of pain and toxicities did not differ between the 2 donation processes for most donors. Among obese donors, PBSC donors were at increased risk of grade 2 to 4 pain as well as grade 2 to 4 toxicities during the pericollection period. In contrast, BM donors were more likely to experience grade 2 to 4 toxicities at 1 week and pain at 1 week and 1 month after the procedure. BM donors experienced slower recovery, with 3% still not fully recovered at 24 weeks, whereas 100% of PBSC donors had recovered. Other factors associated with toxicity included obesity, increasing age, and female sex. In summary, this study provides extensive detail regarding individualized risk patterns of PBSC versus BM donation toxicity, suggesting donor profiles that can be targeted with interventions to minimize toxicity.
Figures


References
-
- Miller JP, Perry EH, Price TH, et al. Recovery and safety profiles of marrow and PBSC donors: experience of the National Marrow Donor Program. Biol Blood Marrow Transplant. 2008;14(9 Suppl):29–36. - PubMed
-
- King RJ, Confer DL, Greinix HT, et al. Unrelated hematopoietic stem cell donors as research subjects. Bone Marrow Transplant. 2011;46(1):10–13. - PubMed
-
- Shaw BE, Ball L, Beksac M, et al. Donor safety: the role of the WMDA in ensuring the safety of volunteer unrelated donors: clinical and ethical considerations. Bone Marrow Transplant. 2010;45(5):832–838. - PubMed
-
- Switzer GE, Harrington D, Haagenson MD, et al. Health-related quality-of-life among adult matched unrelated stem cell donors: a Blood and Marrow Transplant Clinical Trials Network (BMT CTN) randomized trial of marrow versus PBSC donation [abstract]. Blood (ASH Annual Meeting Abstracts) 2010;116(21) Abstract 366.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

