IgG Fab fragments forming bivalent complexes by a conformational mechanism that is reversible by osmolytes
- PMID: 23109335
- PMCID: PMC3522289
- DOI: 10.1074/jbc.M112.410217
IgG Fab fragments forming bivalent complexes by a conformational mechanism that is reversible by osmolytes
Abstract
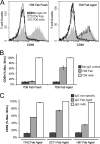
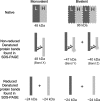
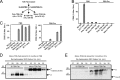
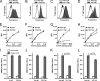
Generated by proteolytic cleavage of immunoglobulin, Fab fragments possess great promise as blocking reagents, able to bind receptors or other targets without inducing cross-linking. However, aggregation of Fab preparations is a common occurrence, which generates intrinsic stimulatory capacity and thwarts signal blockade strategies. Using a panel of biochemical approaches, including size exclusion chromatography, SDS-PAGE, mass spectrometry, and cell stimulation followed by flow cytometry, we have measured the oligomerization and acquisition of stimulatory capacity that occurs in four monoclonal IgG Fabs specific for TCR/CD3. Unexpectedly, we observed that all Fabs spontaneously formed complexes that were precisely bivalent, and these bivalent complexes possessed most of the stimulatory activity of each Fab preparation. Fabs composing bivalent complexes were more susceptible to proteolysis than monovalent Fabs, indicating a difference in conformation between the Fabs involved in these two different states of valency. Because osmolytes represent a class of compounds that stabilize protein folding and conformation, we sought to determine the extent to which the amino acid osmolyte l-proline might impact bivalent Fab complexation. We found that l-proline (i) inhibited the adoption of the conformation associated with bivalent complexation, (ii) preserved Fab monovalency, (iii) reversed the conformation of preformed bivalent Fabs to that of monovalent Fabs, and (iv) separated a significant percentage of preformed bivalent complexes into monovalent species. Thus, Fab fragments can adopt a conformation that is compatible with folding or packing of a bivalent complex in a process that can be inhibited by osmolytes.
Figures




Similar articles
-
Rigid crosslinking of the CD3 complex leads to superior T cell stimulation.Front Immunol. 2024 Aug 30;15:1434463. doi: 10.3389/fimmu.2024.1434463. eCollection 2024. Front Immunol. 2024. PMID: 39281668 Free PMC article.
-
Valency of CD3 binding and internalization of the CD3 cell-surface complex control T cell responses to second signals: distinction between effects on protein kinase C, cytoplasmic free calcium, and proliferation.J Immunol. 1986 Jun 1;136(11):3945-52. J Immunol. 1986. PMID: 3084650
-
Identification of cysteinylation of a free cysteine in the Fab region of a recombinant monoclonal IgG1 antibody using Lys-C limited proteolysis coupled with LC/MS analysis.Anal Biochem. 2006 Aug 15;355(2):165-74. doi: 10.1016/j.ab.2006.05.037. Epub 2006 Jun 15. Anal Biochem. 2006. PMID: 16828048
-
Comparison of the three-dimensional structures of a humanized and a chimeric Fab of an anti-gamma-interferon antibody.J Mol Recognit. 1999 Jan-Feb;12(1):19-32. doi: 10.1002/(SICI)1099-1352(199901/02)12:1<19::AID-JMR445>3.0.CO;2-Y. J Mol Recognit. 1999. PMID: 10398393 Review.
-
Dynamic Aspects of the Immunoglobulin Structure.Immunol Invest. 2019 Nov;48(8):771-780. doi: 10.1080/08820139.2019.1597110. Epub 2019 May 2. Immunol Invest. 2019. PMID: 31044633 Review.
Cited by
-
Antibody-dependent cellular cytotoxicity targeting CD4-inducible epitopes predicts mortality in HIV-infected infants.EBioMedicine. 2019 Sep;47:257-268. doi: 10.1016/j.ebiom.2019.08.072. EBioMedicine. 2019. PMID: 31501077 Free PMC article.
-
Evaluation of the impact of antibody fragments on aggregation of intact molecules via size exclusion chromatography coupled with native mass spectrometry.MAbs. 2024 Jan-Dec;16(1):2334783. doi: 10.1080/19420862.2024.2334783. Epub 2024 Mar 27. MAbs. 2024. PMID: 38536719 Free PMC article.
-
Co-potentiation of antigen recognition: A mechanism to boost weak T cell responses and provide immunotherapy in vivo.Sci Adv. 2015 Oct 2;1(9):e1500415. doi: 10.1126/sciadv.1500415. eCollection 2015 Oct. Sci Adv. 2015. PMID: 26601285 Free PMC article.
-
Osmolytes in vaccine production, flocculation and storage: a critical review.Hum Vaccin Immunother. 2019;15(2):514-525. doi: 10.1080/21645515.2018.1526585. Epub 2018 Oct 12. Hum Vaccin Immunother. 2019. PMID: 30273503 Free PMC article. Review.
-
Expression of the Bacterial Enzyme IdeS Using a GFP Fusion in the Yeast Saccharomyces cerevisiae.Methods Mol Biol. 2023;2674:131-146. doi: 10.1007/978-1-0716-3243-7_9. Methods Mol Biol. 2023. PMID: 37258965
References
-
- Andrew S. M., Titus J. A. (2001) Fragmentation of immunoglobulin G. Curr. Protoc. Immunol., Chapter 2, Unit 2.8 - PubMed
-
- Porter R. R. (1958) Separation and isolation of fractions of rabbit γ-globulin containing the antibody and antigenic combining sites. Nature 182, 670–671 - PubMed
-
- Anderson D. E., Bieganowska K. D., Bar-Or A., Oliveira E. M., Carreno B., Collins M., Hafler D. A. (2000) Paradoxical inhibition of T-cell function in response to CTLA-4 blockade. Heterogeneity within the human T-cell population. Nat. Med. 6, 211–214 - PubMed
-
- Kearney E. R., Walunas T. L., Karr R. W., Morton P. A., Loh D. Y., Bluestone J. A., Jenkins M. K. (1995) Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+ T cells in vivo is dependent on CD28 costimulation and inhibited by CTLA-4. J. Immunol. 155, 1032–1036 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

