CHEK2*1100delC heterozygosity in women with breast cancer associated with early death, breast cancer-specific death, and increased risk of a second breast cancer
- PMID: 23109706
- PMCID: PMC3515767
- DOI: 10.1200/JCO.2012.42.7336
CHEK2*1100delC heterozygosity in women with breast cancer associated with early death, breast cancer-specific death, and increased risk of a second breast cancer
Abstract
Purpose: We tested the hypotheses that CHEK2*1100delC heterozygosity is associated with increased risk of early death, breast cancer-specific death, and risk of a second breast cancer in women with a first breast cancer.
Patients and methods: From 22 studies participating in the Breast Cancer Association Consortium, 25,571 white women with invasive breast cancer were genotyped for CHEK2*1100delC and observed for up to 20 years (median, 6.6 years). We examined risk of early death and breast cancer-specific death by estrogen receptor status and risk of a second breast cancer after a first breast cancer in prospective studies.
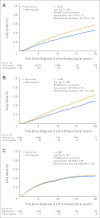
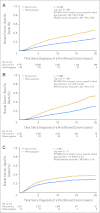
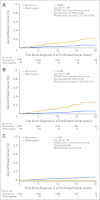
Results: CHEK2*1100delC heterozygosity was found in 459 patients (1.8%). In women with estrogen receptor-positive breast cancer, multifactorially adjusted hazard ratios for heterozygotes versus noncarriers were 1.43 (95% CI, 1.12 to 1.82; log-rank P = .004) for early death and 1.63 (95% CI, 1.24 to 2.15; log-rank P < .001) for breast cancer-specific death. In all women, hazard ratio for a second breast cancer was 2.77 (95% CI, 2.00 to 3.83; log-rank P < .001) increasing to 3.52 (95% CI, 2.35 to 5.27; log-rank P < .001) in women with estrogen receptor-positive first breast cancer only.
Conclusion: Among women with estrogen receptor-positive breast cancer, CHEK2*1100delC heterozygosity was associated with a 1.4-fold risk of early death, a 1.6-fold risk of breast cancer-specific death, and a 3.5-fold risk of a second breast cancer. This is one of the few examples of a genetic factor that influences long-term prognosis being documented in an extensive series of women with breast cancer.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- Weischer M, Bojesen SE, Ellervik C, et al. CHEK2*1100delC genotyping for clinical assessment of breast cancer risk: Meta-analyses of 26,000 patient cases and 27,000 controls. J Clin Oncol. 2008;26:542–548. - PubMed
-
- Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003;3:421–429. - PubMed
-
- Nevanlinna H, Bartek J. The CHEK2 gene and inherited breast cancer susceptibility. Oncogene. 2006;25:5912–5919. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

