Effect of milnacipran on body weight in patients with fibromyalgia
- PMID: 23109813
- PMCID: PMC3479947
- DOI: 10.2147/IJGM.S36444
Effect of milnacipran on body weight in patients with fibromyalgia
Abstract
Background: The purpose of this study was to evaluate the effects of milnacipran on body weight in patients with fibromyalgia.
Methods: ANALYSES WERE CONDUCTED IN THE FOLLOWING GROUPS: patients from three double-blind, placebo-controlled milnacipran trials (3 months, n = 2096; 6 months, n = 1008); 354 patients receiving milnacipran in placebo-controlled trials and double-blind extension studies (total ≥ 12 months of treatment); and 1227 patients in a long-term (up to 3.25 years) open-label milnacipran study.
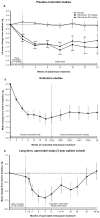
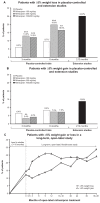
Results: In placebo-controlled trials, 77% of patients were overweight or obese at baseline (body mass index ≥ 25 kg/m(2)). Mean weight loss was found with milnacipran at 3 months (100 mg/day, -1.14 kg; 200 mg/day, -0.97 kg; placebo, -0.06 kg; P < 0.001) and 6 months (100 mg/day, -1.01 kg; 200 mg/day, -0.71 kg; placebo, -0.04 kg; P < 0.05). Approximately twice as many milnacipran-treated patients had ≥5% weight loss from baseline compared with placebo (3 and 6 months, P < 0.01). In extension studies, mean weight loss in patients receiving ≥12 months of milnacipran was -1.06 kg. In patients receiving ≥3 years of treatment in the open-label study, mean changes at 12, 24, 30, and 36-38 months were -1.16, -0.76, -0.19, and +0.11 kg, respectively. Among milnacipran-treated patients, rates of nausea (the most common adverse event) were lower among patients who lost weight than among those who did not (3 months, P = 0.02).
Conclusion: The majority of patients with fibromyalgia in the milnacipran studies were overweight or obese. Milnacipran was associated with mean weight loss at 3 and 6 months (P < 0.05 versus placebo) and at 12 and 24 months of treatment, with mean changes drifting back to baseline at 30 months (-0.19 kg) and 36-38 months (+0.11 kg, no placebo comparison).
Keywords: body weight; fibromyalgia; milnacipran.
Figures

References
-
- Bennett RM. Clinical manifestations and diagnosis of fibromyalgia. Rheum Dis Clin North Am. 2009;35(2):215–232. - PubMed
-
- Lachaine J, Beauchemin C, Landry PA. Clinical and economic characteristics of patients with fibromyalgia syndrome. Clin J Pain. 2010;26(4):284–290. - PubMed
-
- Yunus MB, Arslan S, Aldag JC. Relationship between body mass index and fibromyalgia features. Scand J Rheumatol. 2002;31(1):27–31. - PubMed
LinkOut - more resources
Full Text Sources

