Removing the age restrictions for rotavirus vaccination: a benefit-risk modeling analysis
- PMID: 23109915
- PMCID: PMC3479108
- DOI: 10.1371/journal.pmed.1001330
Removing the age restrictions for rotavirus vaccination: a benefit-risk modeling analysis
Abstract
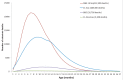
Background: To minimize potential risk of intussusception, the World Health Organization (WHO) recommended in 2009 that rotavirus immunization should be initiated by age 15 weeks and completed before 32 weeks. These restrictions could adversely impact vaccination coverage and thereby its health impact, particularly in developing countries where delays in vaccination often occur.
Methods and findings: We conducted a modeling study to estimate the number of rotavirus deaths prevented and the number of intussusception deaths caused by vaccination when administered on the restricted schedule versus an unrestricted schedule whereby rotavirus vaccine would be administered with DTP vaccine up to age 3 years. Countries were grouped on the basis of child mortality rates, using WHO data. Inputs were estimates of WHO rotavirus mortality by week of age from a recent study, intussusception mortality based on a literature review, predicted vaccination rates by week of age from USAID Demographic and Health Surveys, the United Nations Children's Fund (UNICEF) Multiple Indicator Cluster Surveys (MICS), and WHO-UNICEF 2010 country-specific coverage estimates, and published estimates of vaccine efficacy and vaccine-associated intussusception risk. On the basis of the error estimates and distributions for model inputs, we conducted 2,000 simulations to obtain median estimates of deaths averted and caused as well as the uncertainty ranges, defined as the 5th-95th percentile, to provide an indication of the uncertainty in the estimates. We estimated that in low and low-middle income countries a restricted schedule would prevent 155,800 rotavirus deaths (5th-95th centiles, 83,300-217,700) while causing potentially 253 intussusception deaths (76-689). In contrast, vaccination without age restrictions would prevent 203,000 rotavirus deaths (102,000-281,500) while potentially causing 547 intussusception deaths (237-1,160). Thus, removing the age restrictions would avert an additional 47,200 rotavirus deaths (18,700-63,700) and cause an additional 294 (161-471) intussusception deaths, for an incremental benefit-risk ratio of 154 deaths averted for every death caused by vaccine. These extra deaths prevented under an unrestricted schedule reflect vaccination of an additional 21%-25% children, beyond the 63%-73% of the children who would be vaccinated under the restricted schedule. Importantly, these estimates err on the side of safety in that they assume high vaccine-associated risk of intussusception and do not account for potential herd immunity or non-fatal outcomes.
Conclusions: Our analysis suggests that in low- and middle-income countries the additional lives saved by removing age restrictions for rotavirus vaccination would far outnumber the potential excess vaccine-associated intussusception deaths. Please see later in the article for the Editors' Summary.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, et al. (2011) 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 12: 136–141. - PubMed
-
- WHO (2009) Rotavirus vaccines: an update. Wkly Epidemiol Rec 84: 533–540. - PubMed
-
- Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, et al. (2006) Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 354: 11–22. - PubMed
-
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, et al. (2006) Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 354: 23–33. - PubMed
-
- Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, et al. (2010) Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 376: 606–614. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

