Viral evasion of a bacterial suicide system by RNA-based molecular mimicry enables infectious altruism
- PMID: 23109916
- PMCID: PMC3475682
- DOI: 10.1371/journal.pgen.1003023
Viral evasion of a bacterial suicide system by RNA-based molecular mimicry enables infectious altruism
Abstract
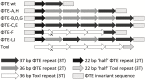
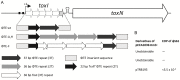
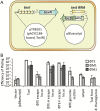
Abortive infection, during which an infected bacterial cell commits altruistic suicide to destroy the replicating bacteriophage and protect the clonal population, can be mediated by toxin-antitoxin systems such as the Type III protein-RNA toxin-antitoxin system, ToxIN. A flagellum-dependent bacteriophage of the Myoviridae, ΦTE, evolved rare mutants that "escaped" ToxIN-mediated abortive infection within Pectobacterium atrosepticum. Wild-type ΦTE encoded a short sequence similar to the repetitive nucleotide sequence of the RNA antitoxin, ToxI, from ToxIN. The ΦTE escape mutants had expanded the number of these "pseudo-ToxI" genetic repeats and, in one case, an escape phage had "hijacked" ToxI from the plasmid-borne toxIN locus, through recombination. Expression of the pseudo-ToxI repeats during ΦTE infection allowed the phage to replicate, unaffected by ToxIN, through RNA-based molecular mimicry. This is the first example of a non-coding RNA encoded by a phage that evolves by selective expansion and recombination to enable viral suppression of a defensive bacterial suicide system. Furthermore, the ΦTE escape phages had evolved enhanced capacity to transduce replicons expressing ToxIN, demonstrating virus-mediated horizontal transfer of genetic altruism.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Comment in
-
Phage biology: phage plays copycat with ToxIN.Nat Rev Microbiol. 2012 Dec;10(12):804-5. doi: 10.1038/nrmicro2920. Epub 2012 Nov 13. Nat Rev Microbiol. 2012. PMID: 23147709 No abstract available.
References
-
- Blower TR, Short FL, Rao F, Mizuguchi K, Pei XY, et al. (2012) Identification and classification of bacterial Type III toxin-antitoxin systems encoded in chromosomal and plasmid genomes. Nucl Acids Res doi:10.1093/nar/gks231 - DOI - PMC - PubMed
-
- Blower TR, Salmond GP, Luisi BF (2011) Balancing at survival's edge: the structure and adaptive benefits of prokaryotic toxin-antitoxin partners. Curr Opin Struc Biol 21: 109–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

