TGFβ1-induced Baf60c regulates both smooth muscle cell commitment and quiescence
- PMID: 23110084
- PMCID: PMC3482188
- DOI: 10.1371/journal.pone.0047629
TGFβ1-induced Baf60c regulates both smooth muscle cell commitment and quiescence
Abstract
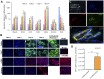
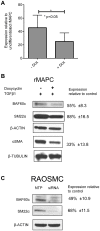
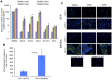
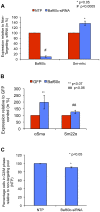
Smooth muscle cells (SMCs) play critical roles in a number of diseases; however, the molecular mechanism underlying their development is unclear. Although the role of TGFβ1 signaling in SMC development is well established, the downstream molecular signals are not fully understood. We used several rat multipotent adult progenitor cell ((r)MAPC) lines that express levels of Oct4 mRNA similar to hypoblast stem cells (HypoSC), and can differentiate robustly to mesodermal and endodermal cell types. TGFβ1 alone, or with PDGF-BB, induces differentiation of rMAPCs to SMCs, which expressed structural SMC proteins, including α-smooth muscle actin (αSMA), and contribute to the SMC coat of blood vessels in vivo. A genome-wide time-course transcriptome analysis revealed that transcripts of Baf60c, part of the SWI/SNF actin binding chromatin remodeling complex D-3 (SMARCD3/BAF60c), were significantly induced during MAPC-SMC differentiation. We demonstrated that BAF60c is a necessary co-regulator of TGFβ1 mediated induction of SMC genes. Knock-down of Baf60c decreased SMC gene expression in rMAPCs whereas ectopic expression of Baf60c was sufficient to commit rMAPCs to SMCs in the absence of exogenous cytokines. TGFβ1 activates Baf60c via the direct binding of SMAD2/3 complexes to the Baf60c promoter region. Chromatin- and co-immunoprecipitation studies demonstrated that regulation of SMC genes by BAF60c is mediated via interaction with SRF binding CArG box-containing promoter elements in SMC genes. We noted that compared with TGFβ1, Baf60c overexpression in rMAPC yielded SMC with a more immature phenotype. Similarly, Baf60c induced an immature phenotype in rat aortic SMCs marked by increased cell proliferation and decreased contractile marker expression. Thus, Baf60c is important for TGFβ-mediated commitment of primitive stem cells (rMAPCs) to SMCs and is associated with induction of a proliferative state of quiescent SMCs. The MAPC-SMC differentiation system may be useful for identification of additional critical (co-)regulators of SMC development.
Conflict of interest statement
Figures

Similar articles
-
Basic fibroblast growth factor antagonizes transforming growth factor-beta1-induced smooth muscle gene expression through extracellular signal-regulated kinase 1/2 signaling pathway activation.Arterioscler Thromb Vasc Biol. 2004 Aug;24(8):1384-90. doi: 10.1161/01.ATV.0000136548.17816.07. Epub 2004 Jun 24. Arterioscler Thromb Vasc Biol. 2004. PMID: 15217807
-
Cytokine-induced differentiation of multipotent adult progenitor cells into functional smooth muscle cells.J Clin Invest. 2006 Dec;116(12):3139-49. doi: 10.1172/JCI28184. Epub 2006 Nov 9. J Clin Invest. 2006. PMID: 17099777 Free PMC article.
-
Platelet-derived growth factor-BB represses smooth muscle cell marker genes via changes in binding of MKL factors and histone deacetylases to their promoters.Am J Physiol Cell Physiol. 2007 Feb;292(2):C886-95. doi: 10.1152/ajpcell.00449.2006. Epub 2006 Sep 20. Am J Physiol Cell Physiol. 2007. PMID: 16987998
-
Programming smooth muscle plasticity with chromatin dynamics.Circ Res. 2007 May 25;100(10):1428-41. doi: 10.1161/01.RES.0000266448.30370.a0. Circ Res. 2007. PMID: 17525382 Review.
-
Origin and differentiation of vascular smooth muscle cells.J Physiol. 2015 Jul 15;593(14):3013-30. doi: 10.1113/JP270033. Epub 2015 Jun 9. J Physiol. 2015. PMID: 25952975 Free PMC article. Review.
Cited by
-
myomiR-dependent switching of BAF60 variant incorporation into Brg1 chromatin remodeling complexes during embryo myogenesis.Development. 2014 Sep;141(17):3378-87. doi: 10.1242/dev.108787. Epub 2014 Jul 30. Development. 2014. PMID: 25078649 Free PMC article.
-
The SWI/SNF subunit SMARCD3 regulates cell cycle progression and predicts survival outcome in ER+ breast cancer.Breast Cancer Res Treat. 2021 Feb;185(3):601-614. doi: 10.1007/s10549-020-05997-5. Epub 2020 Nov 12. Breast Cancer Res Treat. 2021. PMID: 33180234 Free PMC article.
-
Differential requirements for different subfamilies of the mammalian SWI/SNF chromatin remodeling enzymes in myoblast cell cycle progression and expression of the Pax7 regulator.Biochim Biophys Acta Gene Regul Mech. 2022 Feb;1865(2):194801. doi: 10.1016/j.bbagrm.2022.194801. Epub 2022 Feb 23. Biochim Biophys Acta Gene Regul Mech. 2022. PMID: 35217218 Free PMC article.
-
Smad1/5/8 are myogenic regulators of murine and human mesoangioblasts.J Mol Cell Biol. 2016 Feb;8(1):73-87. doi: 10.1093/jmcb/mjv059. Epub 2015 Oct 8. J Mol Cell Biol. 2016. PMID: 26450990 Free PMC article.
-
The multifaceted roles of BAF60 subunits in muscle: regulation of differentiation, reprogramming, and metabolic homeostasis.Front Cell Dev Biol. 2025 Jun 26;13:1594423. doi: 10.3389/fcell.2025.1594423. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40641605 Free PMC article. Review.
References
-
- Drake CJ, Hungerford JE, Little CD (1998) Morphogenesis of the first blood vessels. Ann N Y Acad Sci 857: 155–179. - PubMed
-
- Bergwerff M, Verberne ME, DeRuiter MC, Poelmann RE, Gittenberger-de Groot AC (1998) Neural crest cell contribution to the developing circulatory system: implications for vascular morphology? Circ Res 82: 221–231. - PubMed
-
- Owens GK, Kumar MS, Wamhoff BR (2004) Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84: 767–801. - PubMed
-
- DeRuiter MC, Poelmann RE, VanMunsteren JC, Mironov V, Markwald RR, et al. (1997) Embryonic endothelial cells transdifferentiate into mesenchymal cells expressing smooth muscle actins in vivo and in vitro. Circ Res 80: 444–451. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

