Aspirin inhibits colon cancer cell and tumor growth and downregulates specificity protein (Sp) transcription factors
- PMID: 23110215
- PMCID: PMC3482208
- DOI: 10.1371/journal.pone.0048208
Aspirin inhibits colon cancer cell and tumor growth and downregulates specificity protein (Sp) transcription factors
Abstract
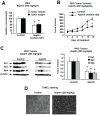
Acetylsalicylic acid (aspirin) is highly effective for treating colon cancer patients postdiagnosis; however, the mechanisms of action of aspirin in colon cancer are not well defined. Aspirin and its major metabolite sodium salicylate induced apoptosis and decreased colon cancer cell growth and the sodium salt of aspirin also inhibited tumor growth in an athymic nude mouse xenograft model. Colon cancer cell growth inhibition was accompanied by downregulation of Sp1, Sp3 and Sp4 proteins and decreased expression of Sp-regulated gene products including bcl-2, survivin, VEGF, VEGFR1, cyclin D1, c-MET and p65 (NFκB). Moreover, we also showed by RNA interference that β-catenin, an important target of aspirin in some studies, is an Sp-regulated gene. Aspirin induced nuclear caspase-dependent cleavage of Sp1, Sp3 and Sp4 proteins and this response was related to sequestration of zinc ions since addition of zinc sulfate blocked aspirin-mediated apoptosis and repression of Sp proteins. The results demonstrate an important underlying mechanism of action of aspirin as an anticancer agent and, based on the rapid metabolism of aspirin to salicylate in humans and the high salicylate/aspirin ratios in serum, it is likely that the anticancer activity of aspirin is also due to the salicylate metabolite.
Conflict of interest statement
Figures

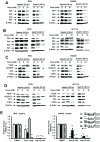

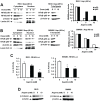
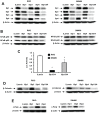
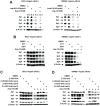

References
-
- Baron JA (2003) Epidemiology of non-steroidal anti-inflammatory drugs and cancer. Prog Exp Tumor Res 37: 1–24. - PubMed
-
- Elwood PC, Gallagher AM, Duthie GG, Mur LA, Morgan G (2009) Aspirin, salicylates, and cancer. Lancet 373: 1301–1309. - PubMed
-
- Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, et al. (2003) A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med 348: 883–890. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

